- Title
-
Characterization of zebrafish pax1b and pax9 in fin bud development
- Authors
- Chen, X., Huang, H., Wang, H., Guo, F., Du, X., Ma, L., Zhao, L., Pan, Z., Gui, H., Yuan, T., Liu, X., Song, L., Wang, Y., He, J., Lei, H., Gao, R.
- Source
- Full text @ Biomed Res. Int.
|
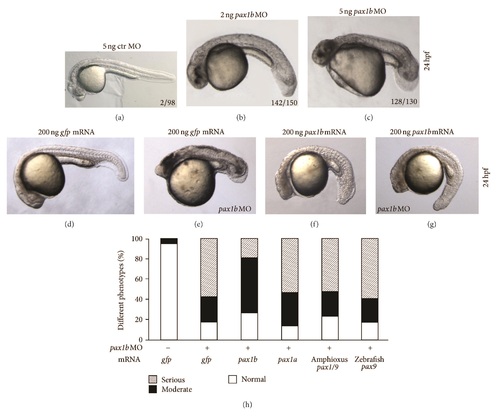
Phenotype caused by pax1b knockdown. In order to analyze the function of pax1b during embryogenesis, we injected pax1b-specific morpholinos into fertilized eggs to block production of functional Pax1b protein. (a) Embryo injected with 5 ng control morpholino. ((b)-(c)) The phenotypes of pax1b morphants caused by 2 ng or 5 ng injection doses. ((d)-(g)) The phenotypes caused by coinjection of 200 ng pax1b or gfp mRNA with 5 ng pax1b MO or control MO. pax1b mRNA could partially rescue the defective fin bud phenotype of the pax1b morphant (g); gfp mRNA failed to rescue defective fin bud phenotype of the pax1b morphant (e). (h) Statistical analysis of phenotypes caused by coinjection of different mRNAs with 5 ng pax1b MO. 150 embryos were calculated. The amount of mRNA injected for every embryo is as follows: 200 ng gfp mRNA, 200 ng pax1b mRNA, 200 ng pax1a mRNA, 150 ng amphioxus pax1/9 mRNA, and 200 ng zebrafish pax9 mRNA. All embryos were observed at 24 hpf. zpax1b: zebrafish pax1b, zpax1a: zebrafish pax1a, zpax9: zebrafish pax9. hpf: hours post-fertilization. gfp: green fluorescence protein. ctr: control. PHENOTYPE:
|
|
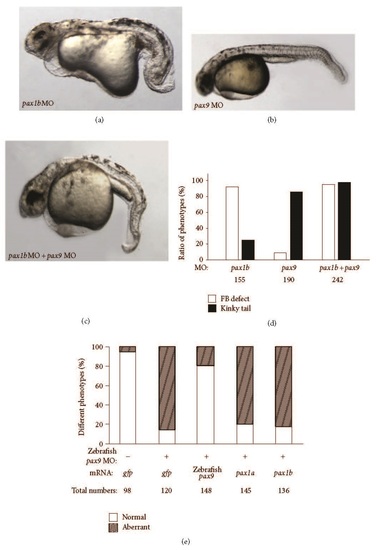
Fin bud and tail defects caused by injection of pax1b MO and/or zebrafish pax9 MO. (a) Fin bud and axis defects caused by injection of 5 ng pax1b MO. (b) Tail defect caused by injection of 5 ng zebrafish pax9 MO. (c) Fin bud and tail defects caused by coinjection of 2.5 ng pax1b MO and 2.5 ng zebrafish pax9 MO. (d) Statistics of phenotype ratios. White columns indicate the ratio of fin bud defect; shaded columns indicate the ratio of kinky tail. (e) Statistical analysis of phenotypes caused by coinjection of different mRNAs with 5 ng zebrafish pax9 MO. The amount of mRNA injected for every embryo is as follows: 200 ng gfp mRNA, 200 ng pax1b mRNA, 200 ng pax1a mRNA, and 200 ng zebrafish pax9 mRNA. All embryos were observed at 36 hpf. PHENOTYPE:
|
|
Fin bud defects caused by pax1b knockdown. ((a)-(d)) Dorsal view of the region near the fin bud with orientation of head towards the top. (a) Control embryos. ((b)-(c)) pax1b morphants. (d) Rescue embryos: coinjection with 5 ng pax1b MO and 200 ng pax1b mRNA. (e) Statistical analysis of fin bud defects caused by injection of control MO or pax1b MO. Total numbers of injected embryos are labeled. ((f)-(m)) Expression pattern of erm and pea3 in control embryos, pax1b morphants, and coinjected embryos. Numbers of defective embryos and total numbers of stained embryos are labeled in the bottoms. The embryonic stage is 72 hpf in panels (a)-(e) and 32 hpf in panels (f)-(m). All the embryos are viewed from the dorsal side with head towards the top. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Molecular mechanism of sclerotome development mediated by Pax1b. (a) The relative expression level of zebrafish collagen type II (col2a1) monitored by qPCR. (b) Sclerotome differentiation analyzed by molecular markers Uncx4.1 and Noggin3 by Western blot. (c) The relative expression level of zebrafish aggrecan monitored by qPCR. The fold change for each group was determined using the delta-delta Ct method. Quantified mRNA levels were normalized to β-actin and are presented relative to control embryos. 50 embryos at the 48 hpf stage were used in each group, performed in triplicate. EXPRESSION / LABELING:
|
|
Forced expression of Pax1 decreases cell death potential on physiological stress. U2OS cells were exposed to 80 J/m2 UV (a) or 2.5 µM doxorubicin (b) for 8 h. Hoechst-positive cells were counted and subjected to statistical analysis using Student’s -tests. All pax1b DNA induction was mediated by the IRES-TOMATO lentivirus system. Data are presented as means ± SE from three independent experiments. *P < 0.05, compared with vector. |