- Title
-
Mosaic analysis and tumor induction in zebrafish by microsatellite instability-mediated stochastic gene expression
- Authors
- Koole, W., and Tijsterman, M.
- Source
- Full text @ Dis. Model. Mech.
|
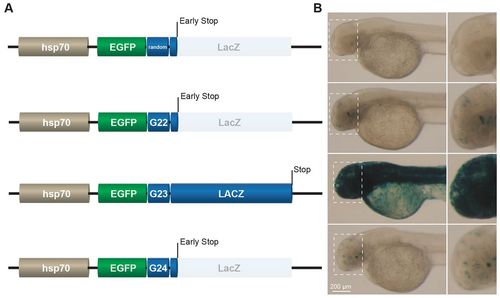
Microsatellite-dependent stochastic gene activation in Danio rerio. (A) Schematic representation of reporters in which the coding sequence of LacZ was placed downstream of an EGFP ORF; a random sequence or a frameshift-prone G22, G23 or G24 microsatellite tract was placed in between these two elements. The random sequence, G22 and G24 put the LacZ ORF out-of-frame. The reporter was under the control of the hsp70 promoter. (B) Images of LacZ-stained embryos (2 dpf) that had been injected with the corresponding reporters shown in A. The embryos show microsatellite-dependent stochastic LacZ expression (blue) in vivo. One-cell stage embryos were rendered transgenic by using Tol2-transposase-mediated transgenesis. Inset images on the right correspond to the dashed box areas shown in the left-hand images. |
|
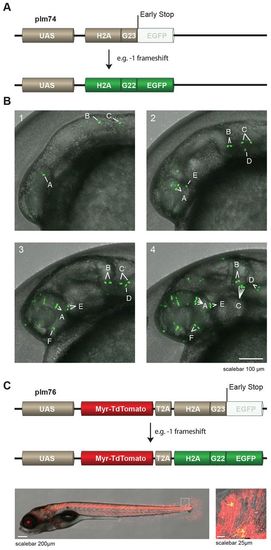
UAS-reporters stochastically express H2A-EGFP. (A) (Upper panel) Schematic representation of reporter plm74 in which frameshifts at a G23 microsatellite can result in EGFP expression. Note that, for illustration purposes, we used the example of a 1 frameshift, however other, bigger, frameshifts can also lead to in-frame EGFP. UAS, upstream activator sequences. (B) Time-lapse images of the head region of an embryo derived from crossing Et(E1b:Gal4-VP16)s1101t fish to Tg(UAS:H2A-G23-EGFP)hu6243 fish. Stochastic expression of nuclear EGFP (green) in cells was observed, and individual cells and their descendants can be traced over time (denoted by the letters A-F in the images). Images 1–4 represent pictures taken around 16, 21, 25 and 31 hpf and correspond to supplementary material Movie 1. (C) (Upper panel) Schematic representation of reporter plm76 in which Myr-TdTomato is placed downstream of a UAS-cassette, followed by a T2A sequence and H2A-G23-EGFP, meaning that EGFP is out-of-frame. Transgenic F1 animals [Et(E1b:Gal4-VP16)s1101t fish crossed to Tg(UAS:Myr-TdTomato-H2A-G23-EGFP)hu6242] exhibit cells that express fluorescent membrane-labeled TdTomato and, in a mosaic pattern caused by in vivo stochastic frameshifting, EGFP-fluorescent nuclei (lower panels, an enlarged image of the boxed area is shown in the right-hand image). |
|
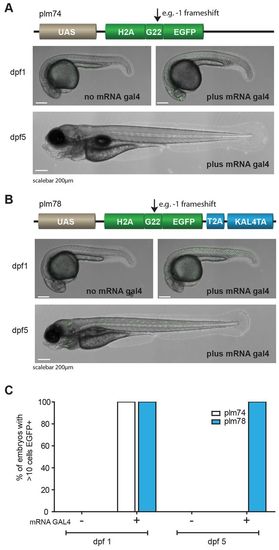
Mosaic labeling with self-maintaining Kaloop. (A) Schematic representation of plm74 (upper panel) in which a 1 frameshift at a G23 microsatellite results in an ORF of H2A-G22-EGFP. The reporter was injected into one-cell stage embryos with or without gal4 mRNA to temporarily activate the reporter. Stochastic expression of nuclear EGFP was observed 1 day after injection (right image) when gal4 mRNA was co-injected, but not in the absence of gal4 mRNA (left image). EGFP expression diminished within 5 days (lower image; quantified in C). (B) Schematic representation of plm78 in which, owing to a 1 frameshift, the coding sequence of EGFP, together with the linker peptide T2A and transcription activator KalTA4, becomes in-frame with the ORF of H2A-G22. Co-injection of gal4 mRNA resulted in stochastic activation of nuclear EGFP in embryos at 1 dpf, and expression was still observed in larvae at 5 dpf. (C) Quantification of injected embryos (n=100 per condition) that express nuclear EGFP. |
|
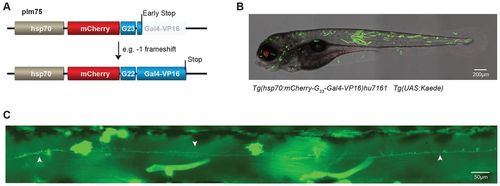
Stochastic activation of Gal4-VP16. (A) (Upper panel) Schematic representation of plm75 in which the coding sequence of Gal4-VP16 is placed downstream of that of mCherry, but out-of-frame because of a G23 intervening sequence, all under the control of the heat shock hsp70 promoter. (B) A representative (merged) image shows stochastic expression of Kaede in an embryo at 5 dpf from a cross of the transgenic line Tg(hsp70:mCherry-G23-Gal4-VP16)hu7161 with Tg(UAS:Kaede). (C) Image of a single neuron that was activated stochastically (indicated with white arrows). |
|
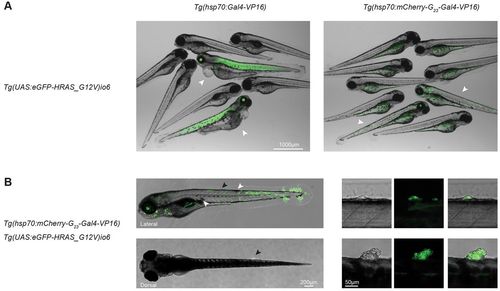
Sporadic tumor induction. (A) Merged images show the difference between the expression of UAS:EGFP-HRAS-G12V in a tissue-wide (left) and stochastic (right) manner in embryos; embryos die at 3 dpf upon tissue-wide overexpression of EGFP-HRAS-G12V using hsp70:Gal4 as an activator, whereas embryos with stochastic activation of EGFP-HRAS-G12V are healthy. White arrowheads indicate embryos that are transgenic for both alleles (the driver and effector alleles), other embryos carried neither of the alleles, or only one of them. (B) Lateral (top images) and dorsal (bottom images) view of a larvae (5 dpf) with stochastic activation of EGFP-HRAS-G12V. Hyperpigmentation (white arrowheads) and tumor formation (black arrowheads) are indicated. Right panels are enlarged images of the areas indicated by the black arrowheads. Brightfield, EGFP and merged channels are displayed. |
|
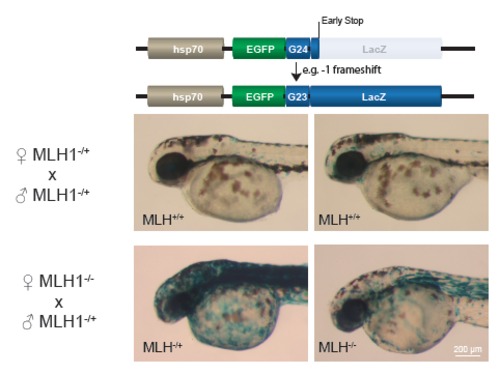
Increased microsatellite instability reporter activation in mismatch repair deficient embryos. Images of embryos (2dpf) stained for LacZ expression. One-cell stage embryos were made transgenic via Tol2-transposase mediated transgenesis with the indicated microsatellite instability construct that reports LacZ expression when the LacZ-coding sequence becomes in-frame after a frameshift. Strong LacZ-staining (examples shown in bottom panels) was seen in 20 out of 22 injected viable embryos that were obtained from a cross between an MLH1-/- mother and an MLH1-/+ father. Genotyping of animals showed that also heterozygous embryos from this cross had strong LacZ-staining, suggesting that the compromised mismatch repair pathway (due to MLH1-deficiency in the yolk) is not rescued by the paternal MLH1 wildtype allele during early development. Injected embryos obtained from MLH1-/+ parents, showed LacZ-staining similar to wild type levels (top panels and Fig. 1). |
|
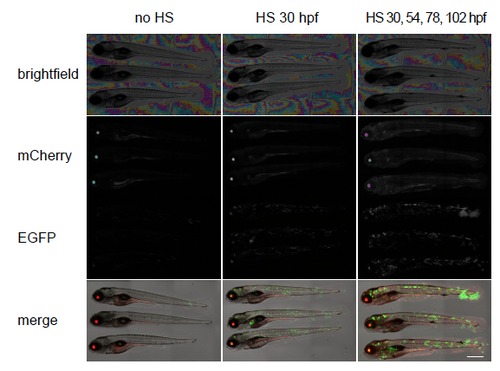
Random activation of oncogenic H-RAS by stochastic Gal4 expression. Larvae were heat shocked (HS) at various time points, as indicated. Larvae were derived from a cross Tg(hsp70:mCherry-G23-Gal4VP16)hu7161 with Tg(UAS:EGFP-H-RAS_G12V)io6. Larvae subjected to heat shock treatment showed stochastic expression of EGFP-H-RAS(G12V), hyperpigmentation and tumor formation. The level of these phenotypes was depended on the number of heat shock treatments (see Table 1 for quantification). Images were taken at 126 hpf. Scalebar indicates 0.5 mm. |