- Title
-
Nkcc1 (Slc12a2) is required for the regulation of endolymph volume in the otic vesicle and swim bladder volume in the zebrafish larva
- Authors
- Abbas, L., and Whitfield, T.T.
- Source
- Full text @ Development
|
lte mutant zebrafish larvae have a collapsed ear at 5 dpf and an increased swim bladder volume. (A-D) Live images of lteto27d homozygous mutant (B,D) and phenotypically wild-type sibling (sib) (A,C) embryos at 5 dpf. (E) Ear collapse in the tg414b allele at 5 dpf is slightly more severe than in to27d. (F-I) Acridine Orange staining (green) indicates that there is no increase in cell death in the ear (white dashed outline) in lte mutants as compared with siblings at 5 dpf (F,H compared with G,I). Background staining in live neuromasts can be seen in both genotypes (green spots). (J-M) FITC-phalloidin (green)/propidium iodide (red) staining reveals similar architecture and number of cells within the otic epithelium of tg414b and siblings at 5 dpf. (L,M) Enlargement of the ventral pillar (white asterisks in J,K). (N,O) Live images showing the increase in swim bladder volume in lteto27d mutants. (P) The average swim bladder length increases from 390 μm in sibling embryos to 418 μm in the mutants (n=12 siblings, n=12 mutants; P=0.03, Student′s t-test). Horizontal lines indicate the distribution of swim bladder lengths; vertical lines indicate the population mean; boxes indicate standard deviation. asc, anterior semicircular canal; psc, posterior semicircular canal; usc, utriculosaccular chamber. Scale bars: 125 μm in A,B; 50 μm in C-E,H-K; 200 μm in F,G; 100 μm in N,O. PHENOTYPE:
|
|
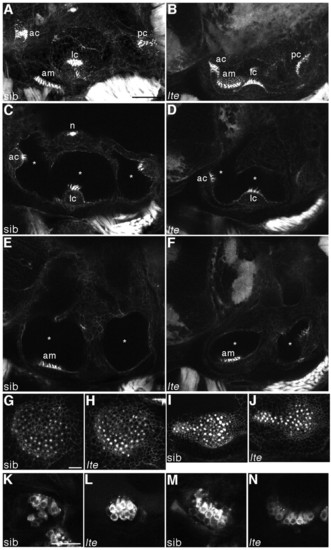
Hair cells do not degenerate and are still functional in lte mutant ears. (A-J) Staining with FITC-phalloidin labels hair cells at 6 dpf. In both siblings (A,C,E,G,I) and lte mutants (lteto27d in B,D,F; ltetg414b in H,J), actin-rich hair bundles are visible in all five sensory patches. Enlargements of the anterior (G,H) and posterior (I,J) maculae show equivalent numbers of differentiated hair cells in siblings and lte mutants. Asterisks (C-F) mark the reduction in the volume of the otic vesicle lumina in the mutant. (K-N) Live staining with FM1-43 marks hair cell endocytosis at 5 dpf, labelling functional cells in the cristae (K,L) and the anterior macula (M,N) in siblings (K,M) and mutants (L,N). A,B,G-J are projections of confocal z-stacks; C-F,K-N are individual optical sections, in which not all sensory patches are visible. am, anterior macula; ac, anterior crista; lc, lateral crista; pc, posterior crista; n, neuromast. Scale bars: 50 μm in A-F; 25 μm in G-N. |
|
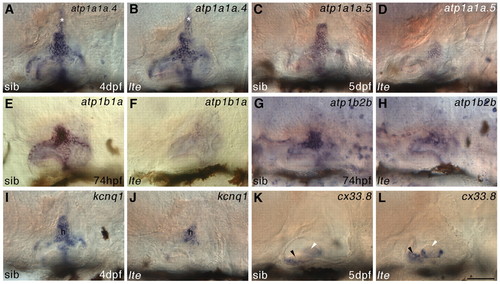
Expression of ionic homeostasis genes is disrupted in the ears of lte mutants. (A-H) Whole-mount in situ hybridisation showing that expression of alpha (a) and beta (b) Na+/K+-ATPase subunit genes is downregulated in the inner ear of lte mutants at 74 hpf (atp1b1a in E,F; atp1b2b in G,H), 4 dpf (atp1a1a.4 in A,B) and 5 dpf (atp1a1a.5 in C,D). The endolymphatic duct (asterisk, A,B) expresses atp1a1a.4 and appears normal in lte. (I,J) At 4 dpf, otic expression of the potassium channel gene kcnq1 is also reduced, mainly in the central semicircular canal hub (h). (K,L) Conversely, expression of connexin 33.8 (cx33.8) at 5 dpf is expanded in the anterior macula of lte mutants (black arrowheads), but remains unchanged in the lateral crista (white arrowheads; out of plane of focus in K). Scale bar: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Expression of nkcc1 transcript and protein immunoreactivity are lost in lte mutants. (A-J) Whole-mount in situ hybridisation for nkcc1 reveals a reduction or loss of expression in lte mutants at all stages examined. (A,F) Four-somite stage (4S). Expression is reduced in the notochord (arrows) and somites (asterisks) in putative mutants. (B-E,G-J) Expression is reduced or lacking in the lte mutant ear from 24 hpf to 5 dpf. (K-V) Immunostaining with the T4 (Nkcc1/2) antibody. Immunoreactivity is lost at 6 dpf in the lte mutant ear (K,L). Expression of Nkcc2 in the pronephric ducts is unaffected in mutants (transverse sections M,N). (O-R) Confocal transverse section through the wild-type ear at 6 dpf; O is a brightfield image and R is an overlay of P and Q. Nkcc1 expression is restricted to the cells lining the medial face of the ventral pillar (P,R, red). TO-PRO-3 staining of nuclei (Q,R, blue) confirms that expression is restricted to the cell membrane and is highest basolaterally (P′). (S-V) Confocal transverse section through the pronephric ducts at 6 dpf showing that Nkcc2 expression is restricted to the apical cell membrane. h, semicircular canal hub; p, pronephric duct; v, ventral pillar; usc, utriculosaccular chamber; lsc, lateral semicircular canal lumen. Scale bars: 200 μm in A,F; 50 μm in B-E,G-L,O-R; 25 μm in M,N; 12.5 μm in S-V. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Genomic sequencing reveals nkcc1 splice site mutations in both lte alleles. (A) ltetg414b is a G→A transition mutation in the splice donor site of exon 22. The resulting transcript lacks 112 bp and is shifted out of frame. (B) Amplification of nkcc1 cDNA with primers flanking the lost exon (see A; F, forward, R, reverse) reveals a 203 bp band in tg414b homozygotes, compound heterozygotes and full-length subcloned tg414b mutant cDNA, as compared with a 315 bp band amplified from the to27d mutant and sibling embryos, full-length wild-type (WT) and to27d subcloned mutant cDNA. (C) NlaIII digest of PCR products generated with primers flanking the lesion in genomic DNA reveals the introduction of a novel NlaIII site in tg414b mutants, cleaving a 53 bp fragment into 40 bp (asterisk) and 13 bp fragments. (D,E) Sequencing of cDNA from the to27d allele reveals a 13 bp insertion between bases 814 and 815 (D), which can be seen in an RT-PCR reaction using primers flanking this sequence (E): a band of 216 bp is present in the to27d line, as compared with the wild-type 203 bp band, amplified here from the tg414b line. (F) Analysis of to27d genomic DNA reveals a T→G transversion mutation in intron 4, introducing a novel splice site 13 bp upstream of the wild-type site. MO indicates the position of the splice-blocking morpholino used in the rescue experiment (see Fig. 6). (G) Theoretical protein topology models (Tusnàdy and Simon, 1998; Tusnàdy and Simon, 2001). The wild-type protein has 12 transmembrane helices; both the N- and C-termini are predicted to be intracellular. The tg414b protein would be truncated after the eleventh transmembrane helix and lose its C-terminal intracellular localisation. In to27d, a severely truncated isoform is predicted, with only three transmembrane helices remaining. E, extracellular; I, intracellular. |
|
The lte phenotype can be rescued with a splice-blocking morpholino. (A-D) One nanogram of an ATG morpholino knocks down Nkcc1 protein expression in the notochord at 26 hpf (B; arrows in enlargement D) as compared with samples injected with 1 ng of a control morpholino (A; arrows in C). This reduction is not maintained at later stages and no phenocopy of the lte mutation was observed. (E-H) The lte phenotype cannot be rescued with a full-length mRNA encoding nkcc1. Ten picograms of Myc-tagged nkcc1 was injected alongside 10 pg gfp mRNA and embryos stained for Myc expression. At 26 hpf, cells are clearly positive for Myc, indicating expression of the tagged protein (F). Expression is extinguished by 74 hpf (H). No Myc expression is detected in embryos injected with gfp RNA alone (E,G). (I-X) A morpholino directed against the ectopic splice site in to27d can rescue the ear collapse phenotype at 6 dpf. Ear size in live injected mutants (K) can become indistinguishable from that of uninjected siblings (I) and is greatly increased compared with uninjected mutants (J). The sequencing traces (N-P,U-X) below each image confirm the genotype of the pictured embryos; the asterisk indicates the position of the mutation (T, wild-type allele; G, mutant allele). Examples of `mild′ and `moderate′ rescued embryos (see Table 1) are depicted in L and M, respectively; a rare complete rescue that would have been scored as `wild type′ (Table 1) is shown in K. Rescue can also be seen at the protein level: T4 staining shows that an injected homozygous mutant (T) has similar levels of protein to an uninjected wild-type sibling (Q) or an injected heterozygous sibling (S). No antibody staining is present in the ear of an uninjected homozygous mutant (R). Scale bars: 100 μm in A-F; 50 μm in G-M,Q-T. |
|
Early patterning of the otic vesicle appears normal in lte mutants. Whole-mount in situ hybridisation shows that the expression of early patterning markers in the otic vesicle is indistinguishable between siblings and mutants (n=30 plus embryos per batch from a cross between heterozygous parents; staining was identical in all embryos within a batch). Normal expression is seen for eya1 ventrally (A), pax2a medially (B) and tbx1 laterally (C) at 25-27 hpf. The statoacoustic ganglion delaminates as usual from the otic vesicle, marked by neurod expression at 28 hpf (D). bmp4 and cahz mark the cristae (E, white asterisks) and maculae (F), respectively, at 52 hpf. The endolymphatic duct, marked by expression of foxi1 (G), appears to evaginate normally. The semicircular canal projections also form normally (H, black asterisks). d, dorsal; l, lateral; m, medial; v, ventral; am, anterior macula; ed, endolymphatic duct; ov, otic vesicle; pm, posterior macula. A,E-H, lateral views; B-D, ventral views. Scale bars: 50 μm. |
|
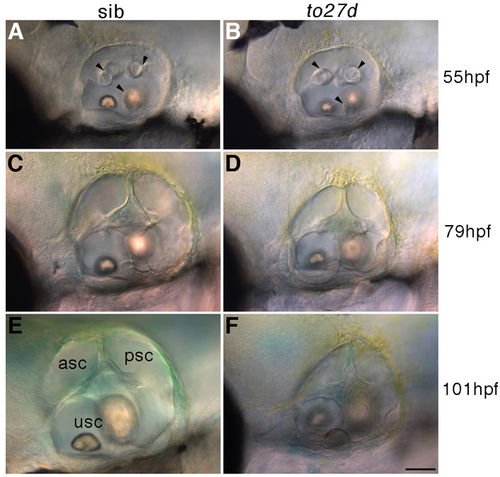
Initiation of ear collapse occurs around 3 dpf in lte mutants. Live DIC pictures of siblings (A,C,E) and to27d mutants (B,D,F) show that the ear sizes are comparable at 55 hpf (A, sib; B, mutant, identified by its ear collapse at 3 dpf) and that the semicircular canal projections are forming normally (black arrowheads). However, by 79 hpf, there is an obvious size reduction in the mutant (D) compared with the sibling (E), which is maintained at 4 dpf (E,H). asc, anterior semicircular canal; psc, posterior semicircular canal; usc, utriculosaccular chamber. Scale bar: 50 μm. |
|
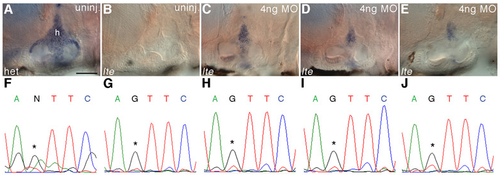
Rescue with a splice-blocking morpholino restores nkcc1 transcript in the ear. (A-E) Whole-mount in situ hybridisation at 6 dpf with an nkcc1 probe shows a graded reappearance of transcript in the ears of to27d embryos injected with 4 ng splice-blocking morpholino, which correlates with the ear size in the live embryo. In the morpholino-rescued mutant embryos, wild-type transcript levels (c.f. uninjected sib, A) are not seen; however, the mild (C), moderate (D) and severe (E) ear volume reduction classes have increased nkcc1 levels when compared with the uninjected mutant (B). All embryos shown were stained under identical conditions and the reaction was stopped simultaneously. (F-J) Confirmation of the genotype of the embryos shown in A-E by sequencing. A is a heterozygous sib (F); B-E are homozygous mutants. Asterisks denote critical nucleotide. h, hub of semicircular canal. Scale bar: 50 μm. |

Unillustrated author statements |