Fig. 5
- ID
- ZDB-FIG-240620-95
- Publication
- Kawano et al., 2022 - NudC regulated Lis1 stability is essential for the maintenance of dynamic microtubule ends in axon terminals
- Other Figures
- All Figure Page
- Back to All Figure Page
|
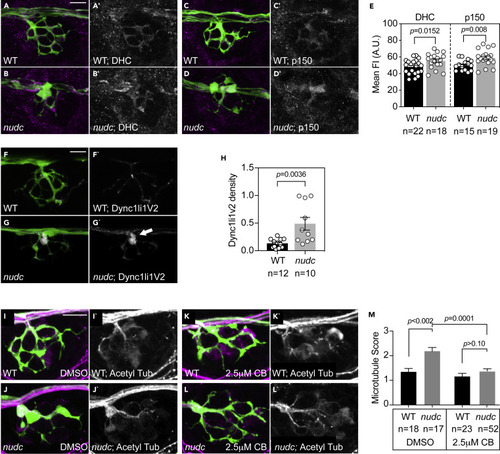
Pharmacological inhibition of dynein suppresses microtubule stabilization in nudc mutant axon terminals (A–D) Dynein heavy chain (DHC) and p150 (dynactin) accumulate in nudc mutant axon terminals. (E) Quantification of mean fluorescence intensity (normalized to background) for DHC and p150 (ANOVA). (F and G) mRFP-Dync1li1V2 expression in individual axons of wild type and nudc mutants demonstrates accumulation in mutant axon terminals (arrow). (H) Quantification of mRFP-Dync1li1V2 density (Dync1li1V2 area/axon terminal area) demonstrates the accumulation of this dynein light intermediate chain in nudc mutant axon terminals (ANOVA). (I–L) Wild type (I) and nudc mutant (J) axon terminals after 24 h 0.1% DMSO treatment. nudc mutants show large axon branchpoint swellings and acetylated tubulin netting in those swellings. (K and L) Treatment with 2.5 mM ciliobrevin (CB) suppresses axonal swellings and microtubule netting in nudc mutants. (M) Average qualitative score from two independent, blinded analyses (scale: 1 - wild-type microtubules; 2 – mild microtubule looping; 3 – presence of microtubule nets; Wilcoxon each pair). Data are expressed as mean ± S.E.M. Scale bar = 10 μm. Sample sizes indicated on graphs. |