Fig. S1
- ID
- ZDB-FIG-110812-9
- Publication
- Paridaen et al., 2011 - The nucleolar GTP-binding proteins Gnl2 and nucleostemin are required for retinal neurogenesis in developing zebrafish
- Other Figures
- All Figure Page
- Back to All Figure Page
|
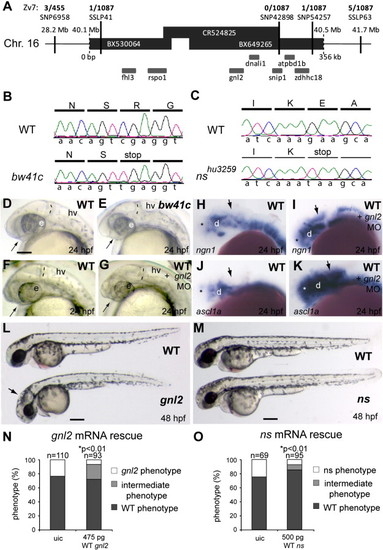
Characterization of gnl2 and ns mutants. (A and B) Positional cloning of the bw41c mutation by mapping of 1087 mutant embryos. Shown is the genomic candidate region, consisting of 3 overlapping bacterial artificial chromosomes (BACs; black bars) that cover about 350 kb (indicated below the bars) of chromosome 16. The position of this region on the chromosome is indicated above the bars. The SNPs and SSLPs used to position the mutation to this region and the numbers of recombinant mutant embryos for these polymorphic markers are indicated above the candidate region. Within the candidate region that is limited by SSLP41 on the left and SNP54257 on the right are 7 genes that are schematically drawn underneath the bars. (B) Sequencing of the bw41c candidate genes revealed the presence of a premature stop codon mutation (cga′tga) at position R53 in exon 3 of guanine nucleotide binding protein-like 2 (gnl2). (C) Using TILLING, a nonsense mutation resulting in a premature stop codon (gaa′taa) at position E117 in exon 5 of guanine nucleotide binding protein-like 3 (gnl3)/nucleostemin (ns) was identified in mutant nshu3259. (D and E) The bw41c mutant (E) phenotype at 24 hpf consists of reduced forebrain (arrow), slightly smaller eyes, thinner mid/hindbrain boundary (MHB; dashed lines) and a slightly hyperinflated hindbrain ventricle. (F–K) Injection of gnl2 translation-blocking morpholino (MO) phenocopies the bw41c mutant phenotype at 24 hpf (F; n = 40) as it causes reduction of the forebrain (arrow) and MHB (dashed line) in combination with a slight reduction of eye size. In addition, injection of gnl2 MO results in expanded expression domains (arrows) of the proneural genes ngn2 (I) and ascl1a (K). (L) WT (top) and bw41c/gnl2 mutant (bottom) embryos at 48 hpf, showing the reduced size of the bw41c/gnl2 embryo, its curved body axis, smaller eye and hyperinflated hindbrain ventricle (arrow). (M) WT (top) and ns mutant (bottom) embryos at 48 hpf, showing the reduced size of the ns head and eyes. (N) Injection of 475 pg WT gnl2 mRNA into progeny of heterozygous carriers results in partial rescue of the mutant morphology in gnl2-/- embryos, characterized by increased size of the total embryo, head and eyes. Number of embryos injected indicated above the bars. (O) Injection of 500 pg WT ns mRNA results in partial rescue of the nshu3295 phenotype. The partial rescue consisted of the increased size of the total embryo, head and eyes. The number of embryos injected is indicated above the bars. Statistics: two-tailed Fisher′s exact probability test for a table of frequency data with p-values indicated above the bar. All images lateral view, anterior to the left and dorsal up. e, eye; hv, hindbrain ventricle; MO, morpholino; uic, uninjected control. Scalebar (C–K) 125 μm, (L, M) 250 μm. |
Reprinted from Developmental Biology, 355(2), Paridaen, J.T., Janson, E., Utami, K.H., Pereboom, T.C., Essers, P.B., van Rooijen, C., Zivkovic, D., and Macinnes, A.W., The nucleolar GTP-binding proteins Gnl2 and nucleostemin are required for retinal neurogenesis in developing zebrafish, 286-301, Copyright (2011) with permission from Elsevier. Full text @ Dev. Biol.