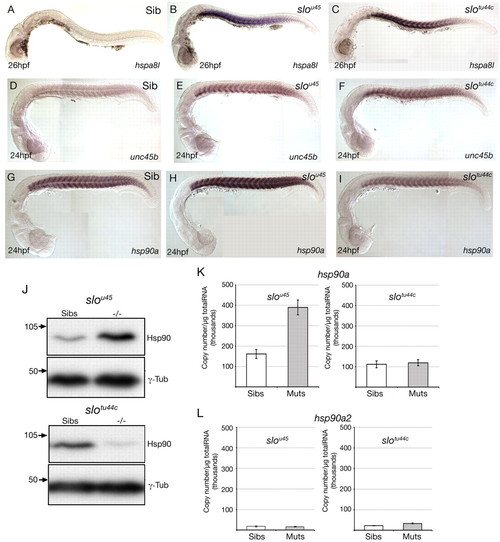
Abrogation of hsp90a but not hsp90a2 function leads to upregulation of genes encoding proteins likely to be involved in sarcomere assembly. (A-I) Lateral views, at the stage indicated bottom left, showing expression of the genes indicated bottom right in wild-type (Sib) and mutant zebrafish embryos. Note that although hspa8l and unc45b are upregulated in the slotu44c allele, hsp90a itself is not. (J) Western blots using lysates of slou45 and slotu44c mutant and sibling embryos. More Hsp90 protein was present in slou45 mutant lysates than in the corresponding siblings. By contrast, slotu44c mutant lysates contained less Hsp90 protein than siblings. (K,L) Quantitative PCR (qPCR) analyses of levels of hsp90a and hsp90a2 expression in wild type (Sibs), slou45 and slotu44c mutants (Muts). Absolute expression levels of hsp90a (normalised to μg of total RNA) were significantly increased (K; P=0.002, n=5) in slou45 mutants compared with siblings; levels of expression of hsp90a in slotu44c mutants were unchanged compared with siblings (K; P=0.75, n=3); and levels of expression of hsp90a2 were not significantly different between slou45mutants and siblings nor between slotu44c mutants and siblings (L; P=0.813, n=5 and P=0.074, n=3, respectively).
|

