Fig. 7
- ID
- ZDB-FIG-050315-5
- Publication
- Hogan et al., 2004 - Zebrafish gcm2 is required for gill filament budding from pharyngeal ectoderm
- Other Figures
- All Figure Page
- Back to All Figure Page
|
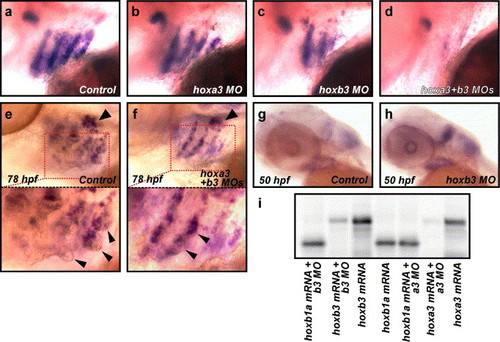
hoxb3, and in its absence hoxa3, are required for normal gcm2 expression. (a–d) Hox group 3 paralogs are required for normal gcm2 expression. Expression of gcm2 in uninjected control embryos at 52 hpf was detected by in situ hybridisation in arches 3–6 (a) and was unchanged in hoxa3 morpholino-injected embryos (b), but was reduced in hoxb3 morpholino-injected embryos (c) and further reduced in hoxa3 + hoxb3 morpholino-injected embryos (d). (e, f) Expression of gcm2 was reduced in hoxa3MO/hoxb3MO morphants in the presence of normal rag1 expression. Expression of gcm2 in distinctive gill filament buds and rag1 in the thymus was detected at 78 hpf in uninjected control embryos (n = 31/35 embryos) (arrowhead in e indicates rag1 expression, lower panel is an enlarged image of boxed section in e, arrowheads in lower panel indicate gill filament buds). Expression of rag1 was also detected in hoxa3MO/hoxb3MO morphants even in the presence of reduced gcm2 expression and reduced gill filament budding (n = 41/71 with reduced gcm2, reduced budding, rag1 positive; n = 14/71 wild type; n = 16/71 with reduced gcm2, reduced budding, rag1 negative) indicating the specificity of the reduced gcm2 phenotype (arrowhead in f indicates rag1 expression, lower panel is an enlarged image of boxed section in f, arrowheads in lower panel indicate gill filament buds). (g, h) Expression of hoxa3 was increased in the absence of hoxb3. Normal expression of hoxa3 was detected by in situ hybridisation in concurrently stained 50 hpf uninjected control embryos (g) and increased intensity of staining was detected in 50 hpf hoxb3MO-injected embryos (n = 34/43) (h). To control against variation in staining, we performed in situ hybridisation on mixed control and MO injected batches, ensuring consistent staining between MO and control injected embryos. We used tail-clipping of experimental groups to provide simple identification of control or MO injected embryos post in situ hybridisation. The identical experiment using random sequence control MO embryos generated n = 33/46 hoxb3MO-injected embryos displaying more intense staining than observed in n = 48 random sequence control MO injected embryos, which was reproduced with hoxB3MOSp (data not shown). (i) Specific activity of hoxa3MO and hoxb3MO, start codon targeted morpholinos assessed by inhibition of in vitro translation assay. hoxb1a mRNA was translated efficiently in the presence of hoxa3 and hoxb3MOs (lanes 1 and 5) and in the absence of MOs (lane 4). Translation efficiency of hoxb3 mRNA in the presence of hoxb3MO (lane 2) represents <15% the translation product seen with hoxb3 mRNA only (lane 3). Translation efficiency of hoxa3 mRNA in the presence of hoxa3MO (lane 6) represents <5% the translation product seen with hoxa3 mRNA only (lane 7). |
| Genes: | |
|---|---|
| Fish: | |
| Knockdown Reagents: | |
| Anatomical Terms: | |
| Stage Range: | Long-pec to Protruding-mouth |
Reprinted from Developmental Biology, 276(2), Hogan, B.M., Hunter, M.P., Oates, A.C., Crowhurst, M.O., Hall, N.E., Heath, J.K., Prince, V.E., and Lieschke, G.J., Zebrafish gcm2 is required for gill filament budding from pharyngeal ectoderm, 508-522, Copyright (2004) with permission from Elsevier. Full text @ Dev. Biol.