- Title
-
Lego Self-Assembly Model of Zebrafish RGB Cone Pentamer Formation: Unique Role of Crumbs2b in Cone Coalescence via Planar Orientational Cell Adhesions
- Authors
- Park, J.S., Guo, C., Wei, X.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
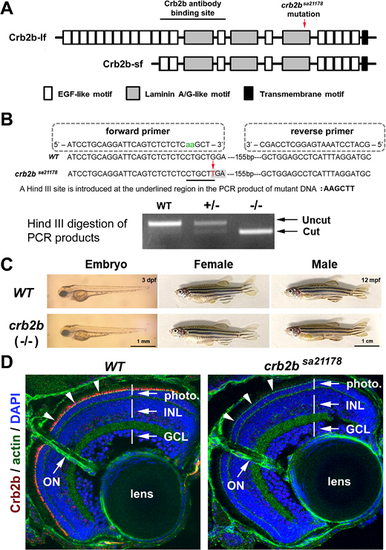
crb2bsa21178 mutation eliminates Crb2b expression in the retina with no gross defects. (A) The position of crb2bsa21178 mutation and the Zou anti-Crb2b antibody binding site in the extracellular domains of the Crb2b-lf and Crb2b-sf. The EGF-like, laminin A/G-like transmembrane motifs are illustrated as shaded boxes. (B) The strategy to genotype wild-type (WT) and crb2bsa21178 mutant (–/–) fish by PCR and Hind III digestion. (C) crb2bsa21178 mutant fish showed no apparent differences in body shape at 3 dpf and adulthood compared to the wild-type. (D) Crb2b immunostaining signals (red, arrowheads) were eliminated in crb2bsa21178 homozygous mutant retina at 5 dpf. The photoreceptor layer (photo), inner nuclear layer (INL), ganglion cell layer (GCL), lens, and optic nerve (ON) were revealed by phalloidin staining of actin and DAPI staining of the cell nuclei. |
|
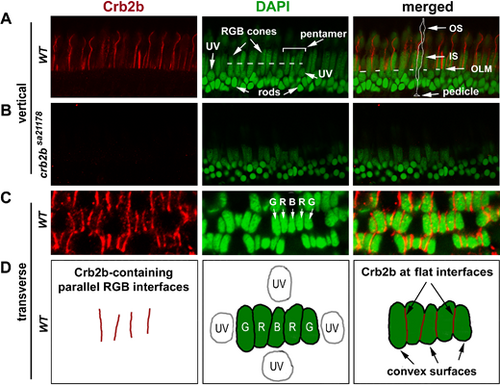
Crb2b localization in the pentamers of RGB cones in 12-mpf retina. (A) Crb2b immunostaining (red) of radial sections of the wild-type photoreceptor layer. The DAPI staining (green) revealed the differences in staining intensities and positioning among the photoreceptor nuclei: lightly stained and more apical positioned long RGB cone nuclei; lightly stained round UV cone nuclei immediately basal to the outer limiting membrane (OLM, the dashed line in the merged panel); and intensively stained smallest rod nuclei at the basal half of the outer nuclear layer. The dashed line in the green panel indicates the position where pentamers were examined at the transverse plane (see panels C, D). A pentamer, flanked by two UV cones, is indicated by a bracket. In the merged image panel, an RGB cone is contoured to show its general polarized morphologies, with its pedicle, inner segment (IS), and outer segment (OS) indicated by arrows. (B) No Crb2b signals (red) were detected in homozygous crb2bsa21178 mutant retinas (radial section). (C, D) Crb2b immunostaining (red) of a transverse section of the photoreceptor layer revealed that Crb2b localized to the junctional interfaces between RGB cones of pentamers, as depicted by the diagrams in D. Note the lack of Crb2b signals at the adjacent convex cell surfaces. |
|
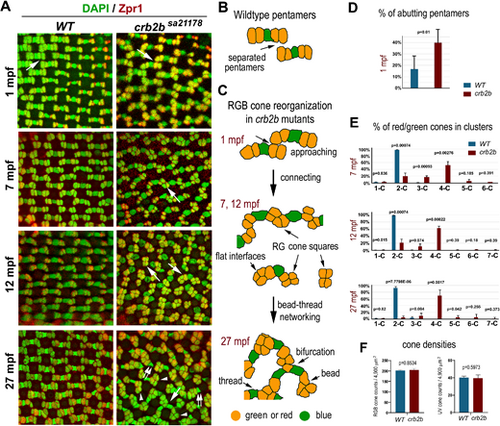
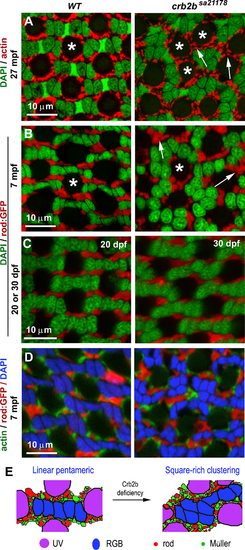
Crb2b deficiency gradually changes the isolated linear mirror-symmetric pentameric configuration of RGB cones into a bead–thread network. (A) Transverse imaging of RGB cones in the wild-type and crb2bsa21178 homozygous mutants of different ages. RGB cone nuclei were stained with DAPI (green), and green and red cones were stained with Zpr1 (red). Large white arrows indicate the groups of RGB cones depicted in panels B and C. Small double arrows indicate blue cones associated with only one cone square; arrowheads indicate square ends with no blue cones attached. (B) The diagram depicts the pentamers in the wild-type retina. (C) Diagrams depict the gradual alteration of the pentameric configuration into a bead–thread network pattern over time in crb2bsa21178 mutants. Orange indicates red or green cones, and green indicates blue cones. The bead–thread networks of RGB cones become apparent even at 7 mpf. Images were taken from the dorsal intermediate regions of the retina. (D) The percentages of abutting pentamers were higher in crb2bsa21178 mutants than those in wild-type retina at 1 mpf (n = 4 fish). (E) The percentages of green and red cones in clusters of various sizes. 1-C indicates isolated single cells; 2-C, two-cell clusters; etc. The percentages were compared between the wild-type and mutants at 7 mpf, 12 mpf, and 27 mpf (n = 4 fish). (F) The densities of RGB and UV cones in the wild-type and crb2bsa21178 homozygous mutants at 27 mpf (n = 4 fish; one tangential confocal image per fish). The P values were calculated by Student's t-test. |
|
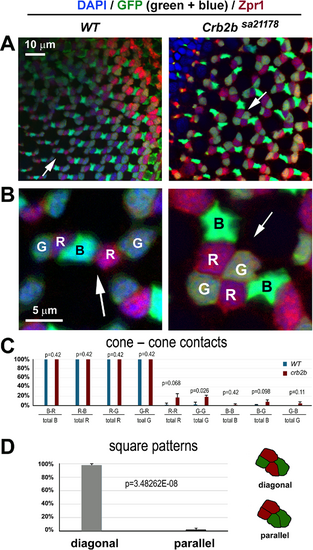
Cones of the same type are positioned diagonally in the red/green cone squares. (A, B) With GFP expression (green) in the green and blue cones of the pt112 transgenic background20 and Zpr1 antibody reactivity (red) in red and green cones, RGB cones can be distinguished unequivocally in the wild-type and crb2bsa21178 homozygous mutants. B shows magnifications of local regions of A (arrows). DAPI staining is blue (at 6 mpf). (C) The preferred cone–cone adhesions are reflected by the percentages of cone types that adhered to themselves or to other types of cones. For example, “R–B/total R” means the percentage of red cones that adhered to one or more blue cones. (D) The distribution of red and green cones in squares in diagonal or parallel patterns, depicted on the right (n = 4 fish for each genotype; one confocal image per fish). P values were calculated with Student's t-test. |
|
Crb2b deficiency results in the disorganization of rods, UV cones, and Müller glial cells, as revealed by transverse imaging of the dorsal intermediate retina. (A) Actin staining (red) revealed that the apical processes of Müller glial cells surround UV cones regularly (asterisks) in the wild-type but often form larger clusters in crb2bsa21178 homozygous mutants. (B, C) Rods, revealed by GFP expression (red) in the Tg(-3.7rho:EGFP)kj2 transgenic background,50 formed linear clusters separating individual pentamers in the wild-type. However, they formed larger irregular clusters in the mutants (arrows) at 7 mpf (B) but not at 1 mpf (C). (D) Simultaneous visualization of actin, rods, and nuclei (DAPI staining) revealed that pentameric cone units were surrounded by rods and Müller glial cell processes. (E) Diagrams summarize the differences in the patterning of all photoreceptor types between the wild-type and crb2bsa21178 homozygous mutant retinas (n = 4 fish for each condition). |
|
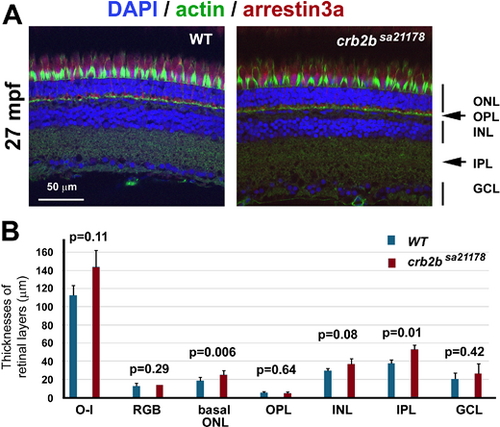
The apicobasal polarization of the retina and photoreceptors remains largely intact in crb2bsa21178 homozygous mutants at 27 mpf. (A) Staining of actin (green, by phalloidin), nuclei (blue, by DAPI), and arrestin-3a (red, by the Zpr1 antibody) retinal layers: ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Images were taken from the central retinal regions. (B) The thicknesses of retinal layers of the wild-type and crb2bsa21178 homozygous mutants. O-I, distance between the outer limiting membrane and the inner limiting membrane; RGB, thickness of the RGB nuclear layer; basal ONL, thickness of the outer nuclear layer region that is basal to the outer limiting membrane. Student's two-tailed and paired t-tests were used to compare the differences in retinal layer thicknesses between the wild-type and mutant samples (n = 4 fish). |
|
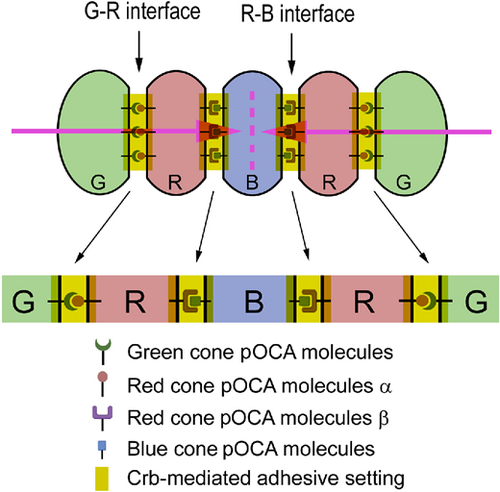
The Lego self-assembly model for RGB cone pentamer formation and maintenance. Diagrams illustrate the hypothesized planar OCAs (pOCAs) that mediate G–R and R–B coalescence. Each type of RGB cone expresses a unique set of pOCA molecules. Blue-cone-specific pOCA molecules are distributed to two opposing parallel surfaces, where they bind to red-cone-specific pOCA molecules β to recruit two red cones in parallel flanking positions. Red cones also express another set of red-cone-specific pOCA molecules α, which localize opposite the pOCA molecules β and bind to green-cone-specific pOCA molecules, thus recruiting a green cone on the side opposite from the central blue cone. These parallel, anisotropically distributed, and cell-type-specific OCAs ultimately dictate the pentameric configuration. Crb2b localizes to all of these intercellular interfaces to play two roles: First, it assists in general adhesion by augmenting the adhesion between pentamer members; second, it helps to restrict G–R cone-specific pOCAs to the parallel junctional interfaces to prevent them from expanding to other membrane regions, which would result in square aggregation of green and red cones of neighboring pentamers. RGB cones are color coded; various OCA molecules are symbolized with distinct icons. The pink arrows indicate the opposing planar polarity within a pentamer, and the pink dashed line indicates the axis of the mirror symmetry of the planar polarities within a pentamer. |