- Title
-
Synergistic and independent roles for Nodal and FGF in zebrafish CPC migration and asymmetric heart morphogenesis
- Authors
- Gonzalez, V., Grant, M.G., Suzuki, M., Christophers, B., Williams, J.R., Burdine, R.D.
- Source
- Full text @ Development
|
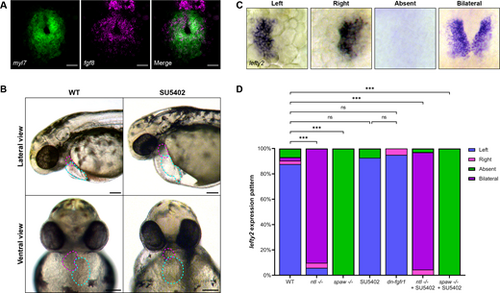
FGF signaling is active in the developing heart and necessary for proper heart development. (A) RNA ISH by hybridization chain reaction for fgf8 (magenta) and myl7 (green) in the cardiac cone of a 20 hpf WT embryo. Scale bars: 25 µm. (B) Representative images of 48 hpf WT embryos or embryos treated with the FGF inhibitor SU5402 from 19 to 30 hpf. Magenta dashed lines outline the ventricle, cyan dashed lines the atrium. SU5402-treated embryos display aberrant cardiac looping, misshapen and misplaced chambers, and pericardial edema. (C) ISH for the Nodal target gene lefty2 (lft2) in the cardiac cone of 20 hpf embryos shows correct left-sided lefty2 and incorrect right, bilateral or absent lefty2 expression. (D) Quantification of lefty2 expression sidedness. WT, n=74; ntl−/−, n=50; spaw−/−, n=36; SU5402, n=41; dn-fgfr1, n=20; ntl−/−+SU5402, n=177; spaw−/−+SU5402, n=63. ***P<0.0001 (Chi-square analysis). ns, not significant. (A,C) Images are dorsal views, with anterior to the top and left to the reader's left. |
|
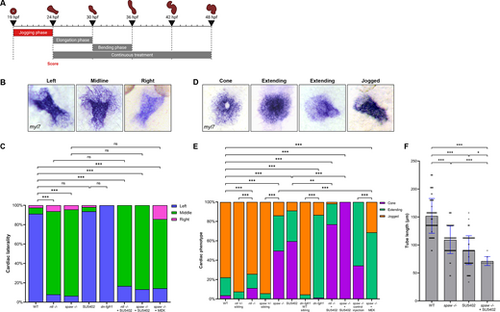
FGF signaling is necessary for proper CPC migration but not cardiac laterality during jogging. (A) Schematic depicting the 19-24 hpf SU5402 treatment window during the ‘jogging phase’. (B) RNA ISH for myl7 at 26.5 hpf visualizing correct left-sided positioning and incorrect midline and right-sided positioning of the heart tube. (C) Quantification of cardiac laterality phenotypes. WT, n=534; ntl−/−, n=113; spaw−/−, n=138; SU5402, n=112; dn-fgfr1, n=75; ntl−/−+SU5402, n=12; spaw−/−+SU5402, n=30; spaw−/−+MEK, n=14. Chi-square analysis. (D) RNA ISH for myl7 at 24 hpf displaying the stages from cone through extending to correctly jogged heart tubes observed in treated embryos. (E) Quantification of cardiac migration phenotypes. WT, n=686; ntl+/− sibling, n=213; ntl−/−, n=152; spaw+/− sibling, n=87; spaw−/−, n=171; SU5402, n=531; dn-fgfr1 WT sibling, n=66; dn-fgfr1, n=225; ntl−/−+SU5402, n=117; spaw−/−+SU5402, n=146; spaw−/− control injection, n=105; spaw−/−+MEK, n=35. Chi-square analysis. (F) Quantification of heart tube length at 26.5 hpf. WT, n=84; spaw−/−, n=62; SU5402, n=80; spaw−/−+SU5402, n=9. Statistical significance determined by an unpaired two-tailed Student's t-test. (B,D) Images are dorsal views, with anterior to the top and left to the reader's left. *P<0.05, **P<0.001, ***P<0.0001. ns, not significant. Error bars represent s.d. |
|
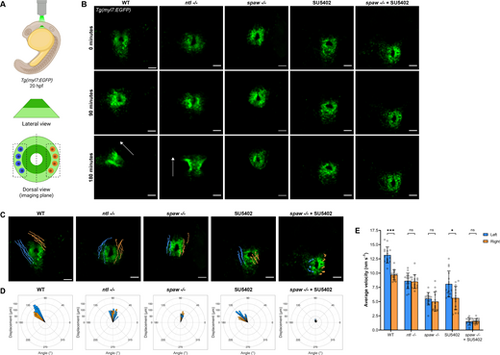
FGF signaling promotes CPC migration while Nodal promotes CPC migration and laterality during jogging. (A) Schematic of the imaging strategy for time-lapse imaging of the cardiac cone throughout jogging, with outer atrial cells in light green and inner ventricular cells in dark green. Left-sided (blue) and right-sided (orange) cells that underwent tracking for analysis are shown. (B) Representative time-lapse images depicting CPC migration from the formation of the cardiac cone through the next 3 h of development. WT and ntl−/− embryos typically complete jogging and tube formation in this time frame. Arrows indicate heart tube direction. Scale bars: 50 µm. (C) Trajectories of left-sided (blue) and right-sided (orange) CPCs during 3 h of jogging. Scale bars: 50 µm. (D) Rose plots demonstrating the angle of displacement of each tracked left-sided (blue) and right-sided (orange) CPC after 3 h of jogging. (E) Average velocity of left-sided and right-sided CPCs during 3 h of jogging. *P<0.05, ***P<0.0001 (unpaired two-tailed Student's t-test). ns, not significant. (C-E) n=3 embryos (five left-sided and five right-sided cells tracked per embryo) per condition. Error bars represent s.d. (B,C) Images are dorsal views, with anterior to the top and left to the reader's left. |
|
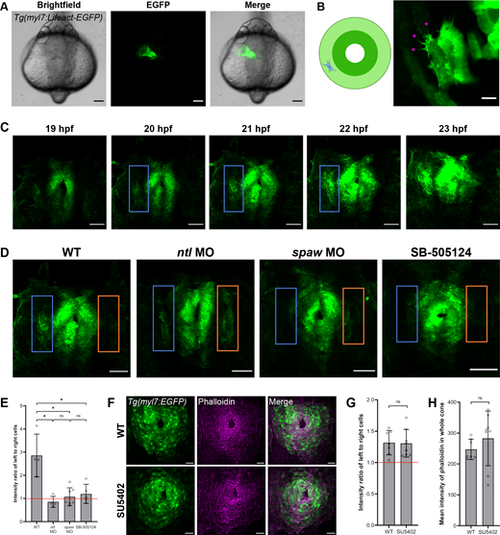
F-actin dynamics are asymmetric and Nodal dependent in CPCs during jogging. (A) Tg(myl7:Lifeact-EGFP) embryo at 24 hpf demonstrating the fluorescence of the CPCs composing the heart tube. (B) Right: F-actin protrusions (pink asterisks) in a single CPC of a mosaic Tg(myl7:Lifeact-EGFP) embryo at 21 hpf. Left: Schematic showing the location of the CPC in the cardiac cone. (C) F-actin protrusive activity in left (blue box) compared to right CPCs in the cardiac cone of a WT Tg(myl7:Lifeact-EGFP) embryo throughout jogging from 19 to 23 hpf. (D) Cardiac cones of Tg(myl7:Lifeact-EGFP) WT, ntl morphant, spaw morphant, and SB505124-treated embryos, with left-sided (blue box) and right-sided (orange box) atrial CPCs outlined. (E) Fluorescence intensity ratio between left-sided and right-sided CPCs in the Tg(myl7:Lifeact-EGFP) embryos. WT, n=4; ntl MO, n=4; spaw MO, n=7; SB-505124, n=5. (F) Cardiac cones of Tg(myl7:EGFP) WT and SU5402-treated embryos stained with phalloidin. (G) Fluorescence intensity ratio between left-sided and right-sided CPCs in the phalloidin-stained embryos. WT, n=7; SU5402, n=8. (H) Fluorescence intensity measurements of the whole cardiac cone in the phalloidin-stained embryos. WT, n=7; SU5402, n=8. (A-D,F) Images are dorsal views, with anterior to the top and left to the reader's left. *P<0.01 (unpaired two-tailed Student's t-test). ns, not significant. Error bars represent s.d. Scale bars: 100 µm (A); 2.5 µm (B); 50 µm (C,D); 25 µm (F). |
|
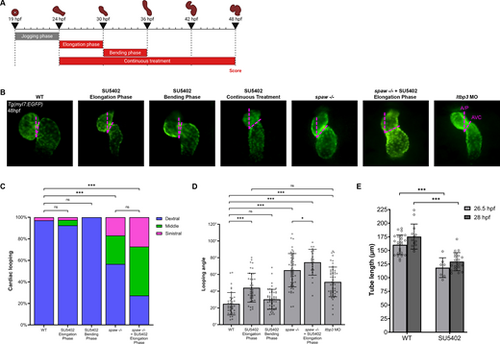
FGF signaling is necessary for SHF addition and proper cardiac looping. (A) Schematic depicting the SU5402 treatment windows during the ‘elongation phase’ from 24 to 30 hpf, the ‘bending phase’ from 30 to 36 hpf, and the ‘continuous treatment’ window from 24 to 48 hpf. (B) Representative images of WT, SU5402-treated, spaw−/− and ltbp3 morphant Tg(myl7:EGFP) embryo hearts at 48 hpf, depicting how the looping angle is measured (pink dashed lines). A/P, anterior/poster axis line; AVC, atrioventricular canal. (C) Quantification of looping laterality phenotypes. WT, n=70; SU5402 elongation phase, n=40; SU5402 bending phase, n=50; spaw−/−, n=53; spaw−/−+SU5402 elongation phase, n=22. Chi-square analysis. (D) Quantification of looping angle. WT, n=38; SU5402 elongation phase, n=39; SU5402 bending phase, n=50; spaw−/−, n=53; spaw−/−+SU5402 elongation phase, n=22; ltbp3 MO, n=50. Unpaired two-tailed Student's t-test. (E) Quantification of heart tube length in WT and SU5402-treated Tg(nkx2.5:ZsYellow) embryo heart tubes at 26.5 or 28 hpf. WT 26.5 hpf, n=24; SU5402 26.5 hpf, n=7; WT 28 hpf, n=13; SU5402 28 hpf, n=19. Unpaired two-tailed Student's t-test. In B, images are ventral views, with anterior to the top and left to the reader's right. *P<0.05, ***P<0.0001. ns, not significant. Error bars represent s.d. |
|
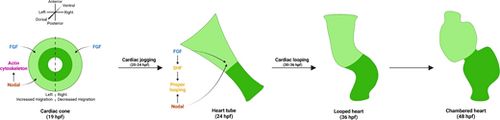
Model of the synergistic and distinct roles of Nodal and FGF signaling in asymmetric cardiac morphogenesis. In the cardiac cone, Nodal promotes a left-lateralized migration to generate cardiac asymmetry, while FGF promotes a permissive migration in all CPCs. Nodal signaling also impinges on the actin cytoskeleton to promote F-actin asymmetry. Next, in the heart tube, both Nodal and FGF signals promote proper cardiac looping. Nodal promotes looping laterality to correctly position the chambers, while FGF induces the SHF which, in turn, is likely necessary for robust looping. The looped heart then matures to form a two-chambered heart by 48 hpf. |