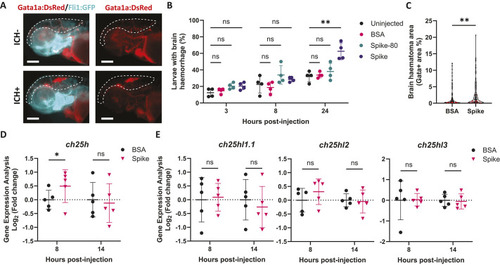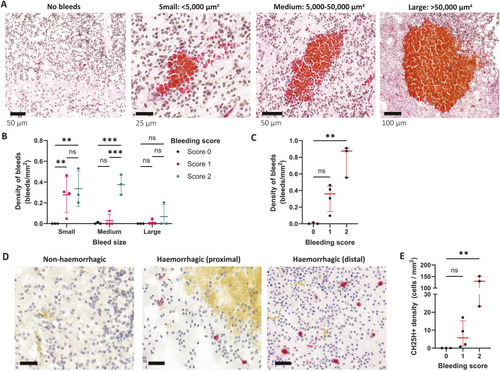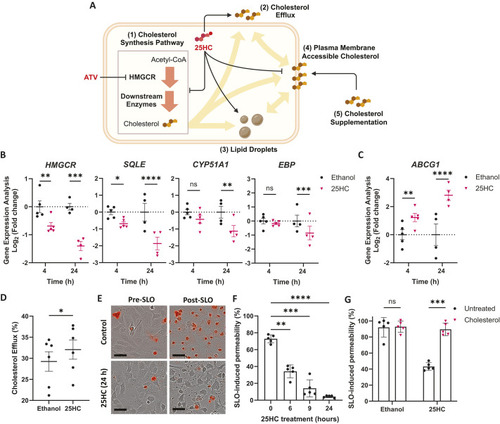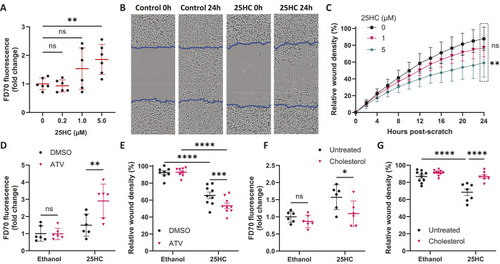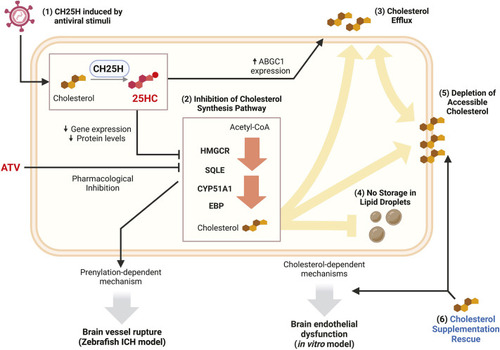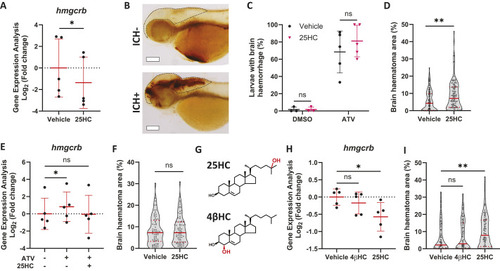
25HC worsens brain haemorrhage expansion in a statin-induced ICH zebrafish model. (A) hmgcrb expression in 2 days post-fertilisation (dpf) wild-type (WT) zebrafish larvae incubated with 25-hydroxycholesterol (25HC; 25 μM, 24 h) (15 embryos pooled per replicate). (B-D) WT larvae were incubated in Atorvastatin (ATV; 1 µM) at 28 h post-fertilisation (hpf) and intravenously injected with 25HC (1 nl, 5 µM) at 32-36 hpf. The next day, larvae were stained with o-Dianisidine. Representative images of larvae without (ICH−) and with (ICH+) brain haemorrhage are shown (B). Dotted lines indicate the brain area. Scale bars: 150 µm. ICH+ frequency per experiment (C) and brain haematoma area per larvae (D) were quantified. Individual embryos are indicated as dots (n=128 embryos, five independent experiments). (E) hmgcrb expression in 2 dpf WT larvae incubated with 25HC (25 µM) and ATV (1 µM) for 24 h (15 embryos pooled per replicate). (F) bbh zebrafish larvae were injected with 25HC (1 nl, 5 µM) at 32-36 hpf. After 24 h, larvae were stained with o-Dianisidine and brain haematoma area was quantified. Individual embryos are indicated as dots (n=69-82 embryos, three independent experiments). (G) Comparison of 25HC and 4β-hydroxycholesterol (4βHC) structures. (H) Expression of hmgcrb in 2 dpf WT larvae incubated with 4βHC or 25HC (25 µM, 24 h) (15 embryos pooled per replicate). (I) WT larvae were incubated in ATV (1 µM) at 28 hpf and injected with 4βHC or 25HC (1 nl, 2.5 µM) at 32-36 hpf. The next day, larvae were stained with o-Dianisidine and haematoma area was quantified. Individual embryos are indicated as dots (n=87-93 embryos, three independent experiments). Data expressed as mean±s.d. (A,C,E,H) or median±IQR (D,F,I). ns, nonsignificant; *P<0.05; **P<0.01; determined by paired two-tailed t-test (A), randomised block two-way ANOVA with Sidak's post-hoc test compared to control (C), Mann–Whitney test (D,F), randomised block one-way ANOVA with Dunnett's post-hoc test compared to control (E,H), or Kruskal–Wallis test with Dunn's post-hoc test compared to control (I).
|