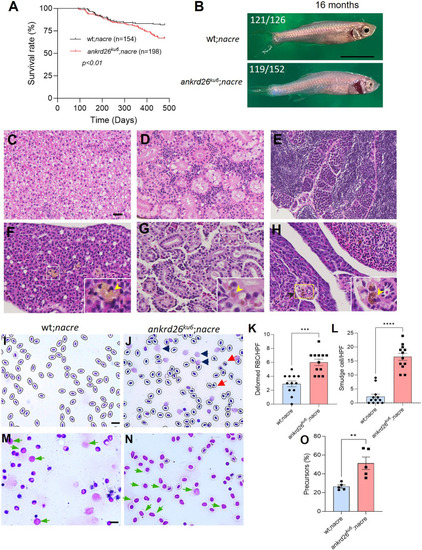
Survival and histological analyses of tissues, blood and kidney marrow of zebrafish of various genotypes. (A) Kaplan–Meier survival analysis indicates the survival rates of wt;nacre (n=154) and ankrd26ku6;nacre (n=198) zebrafish over a 16-month observation. (B) Representative gross morphology in wt;nacre and ankrd26ku6;nacre zebrafish at the age of 16 months. The proportions of abnormal body shapes are indicated at the top left in images. (C-H) Hematoxylin and Eosin staining indicates the absence (C-E) and presence (F-H) of lipofuscin deposition in wt;nacre and ankrd26ku6;nacre zebrafish, respectively, at the age of 16 months, in the liver (C,F), kidney (D,G) and spleen (E,H). Yellow arrowheads indicate representative lipofuscin deposition in the organ tissues of ankrd26ku6;nacre zebrafish. (I,J) Giemsa staining of peripheral blood smears from wt;nacre (I) and ankrd26ku6;nacre (J) zebrafish. Red and black arrowheads in J indicate deformed red blood cells and smudge cells, respectively. (K,L) Quantitation of the deformed red blood cells (K) and smudge cells (L) in peripheral blood smears of wt;nacre and ankrd26ku6;nacre zebrafish. HPF, high-power field; RBC, red blood cells. (M,N) Representative images of stained kidney marrow cells in wt;nacre (M) and ankrd26ku6;nacre (N) zebrafish. Green arrows indicate the increased number of myeloid progenitor cells in ankrd26ku6 zebrafish. (O) Quantitation of myeloid progenitor cells in kidney marrow smears from wt;nacre and ankrd26ku6;nacre zebrafish at the age of 16 months. Data in K, L and O are individuals, means (bars) and s.e.m. (horizontal lines). Mann–Whitney U-test was performed to determine the statistical significance of differences between the two groups. **P<0.01, ***P<0.005, and ****P<0.0001. Scale bars: 10 µm (C,I,M); 1 cm (B).
|