- Title
-
Maternal Exposure to Environmentally Relevant Concentrations of Tris(2,4-di-tert-butylphenyl) Phosphate-Induced Developmental Toxicity in Zebrafish Offspring via Disrupting foxO1/ripor2 Signaling
- Authors
- Zhang, Y., Qin, H., Zu, B., Yu, Z., Liu, C., Shi, J., Zhou, B.
- Source
- Full text @ Env. Sci. Tech.
|
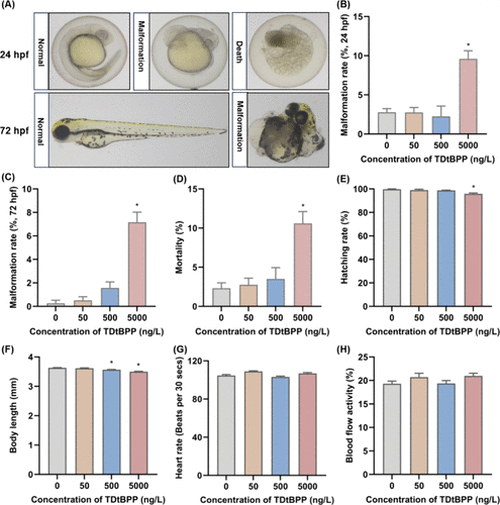
Representative images of normal, malformed, and dead morphology of offspring zebrafish embryos at 24 and 72 hpf (A), malformation rate of offspring zebrafish embryos at 24 and 72 hpf (B, C), mortality (D), hatching rate (E), body length (F), heart rate (G), and blood flow activity (H) of offspring zebrafish embryos at 72 hpf, following maternal exposure to 0, 50, 500, or 5000 ng/L TDtBPP. n = 3. *p < 0.05 was considered to have a statistically significant difference. |
|
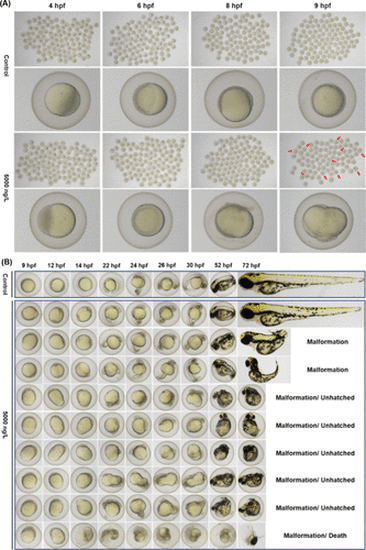
Representative images of developing offspring zebrafish embryos at 4, 6, 8, and 9 hpf (A), and the morphological characteristics of normal (Control) and abnormal (5000 ng/L) epibolic embryos at different time points from 9 to 72 hpf (B), following maternal exposure to 0 or 5000 ng/L TDtBPP. Abnormal epiboly in embryos was indicated by red arrow. |
|
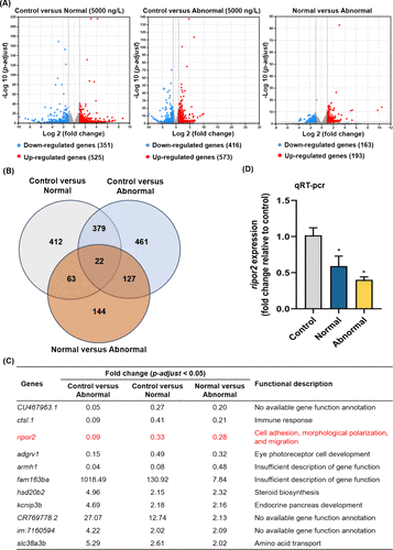
Numbers of significant differently expressed genes in 9 hpf offspring zebrafish embryos between the control (normal epibolic embryos) and 5000 ng/L (normal and abnormal epibolic embryos) (A, n = 3). Up- and down-regulated genes were indicated by red and blue dots, respectively. Venn diagram of these significant differently expressed genes in offspring embryos (B). Concurrently up- or down-regulated genes were identified across three comparisons: control versus normal (5000 ng/L), control versus abnormal (5000 ng/L), and normal versus abnormal both in 5000 ng/L, as well as the fold changes of expression levels and functional descriptions of these genes (C). Expression of ripor2 in 9 hpf offspring embryos in the control and 5000 ng/L (normal and abnormal) groups (D, n = 6, two technical replicates for each experimental replicate). |
|
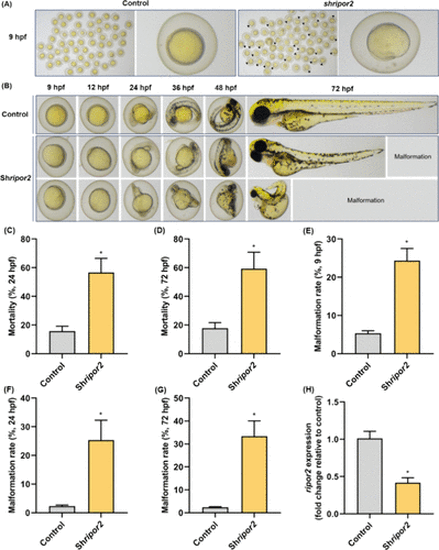
Representative images of developing zebrafish embryos at 9 hpf following microinjection with empty vector or shripor2 (A). Abnormal epiboly in embryos was indicated by a black arrow. Morphological characteristics of normal (empty vector injection) and abnormal (shripor2 injection) epibolic embryos at different time points from 9 to 72 hpf (B). Mortality of injected zebrafish embryos at 24 hpf (C) and 72 hpf (D). Malformation rates of injected zebrafish embryos at 9 hpf (E), 24 hpf (F), and 72 hpf (G). Expression of ripor2 in 9 hpf embryos following injection with an empty vector or shripor2 (H), n = 5. *p < 0.05 was considered to have a statistically significant difference. |
|
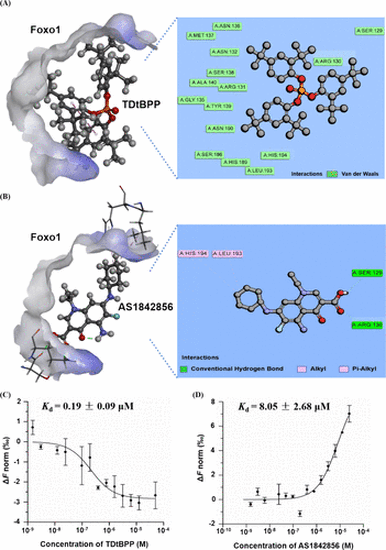
Diagram of docking of zebrafish FoxO1 protein with TDtBPP (A) and AS1842856 (B). Concentration–response curves and measurements of Kd of TDtBPP (C) and AS1842856 (D) with FOXO1 protein interaction. |
|
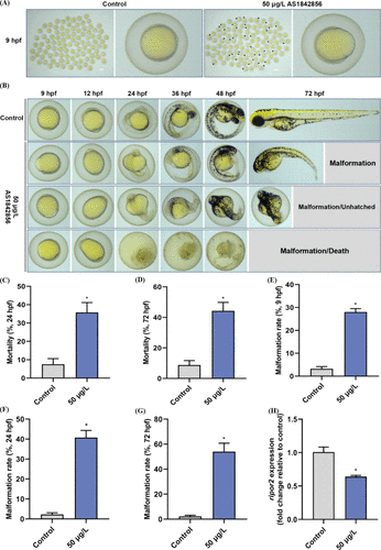
Representative images of developing zebrafish embryos at 9 hpf after exposure to 0 or 50 μg/L AS1842856 (A). Abnormal epiboly in embryos was indicated by black arrow. Morphological characteristics of normal (control) and abnormal (50 μg/L AS1842856) epibolic embryos at different time points from 9 to 72 hpf (B). Mortality of zebrafish embryos at 24 hpf (C) and 72 hpf (D), malformation rate of zebrafish embryos at 9 hpf (E), 24 hpf (F), and 72 hpf (G) after exposure to 0 or 50 μg/L AS1842856, n = 3. Expression of ripor2 in 9 hpf embryos following exposure to 0 or 50 μg/L AS1842856 (H, n = 6, two technical replicates for each experimental replicate). *p < 0.05 was considered to have a statistically significant difference. |