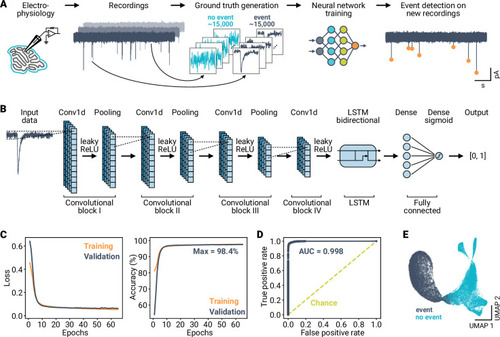
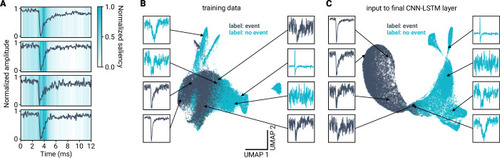
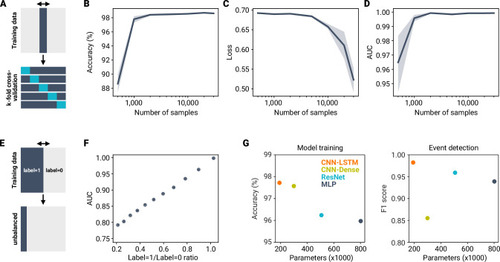
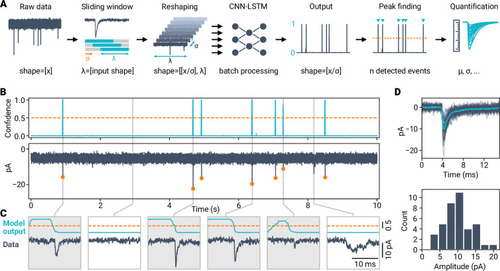
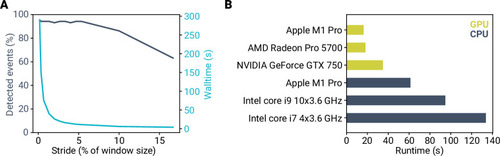
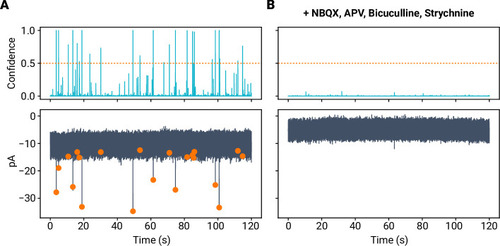
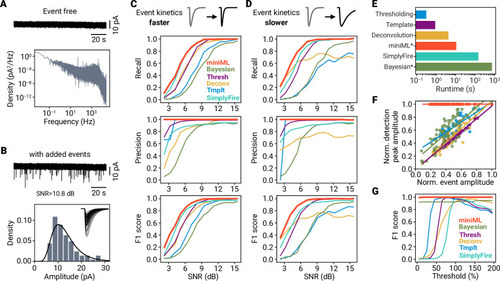
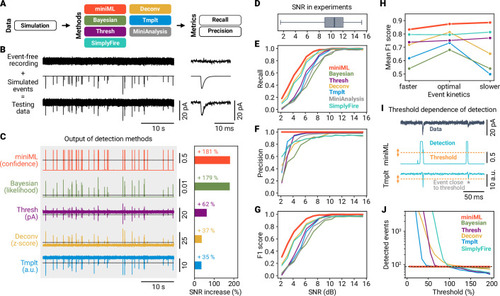
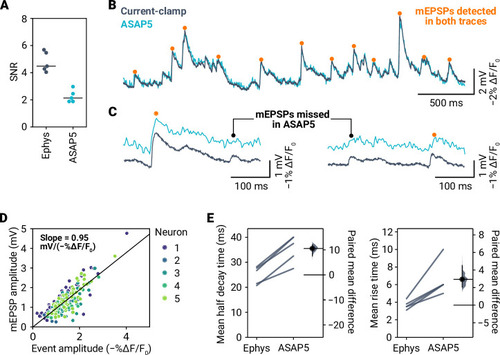
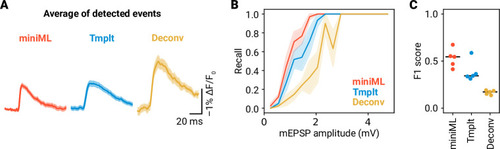
- Title
-
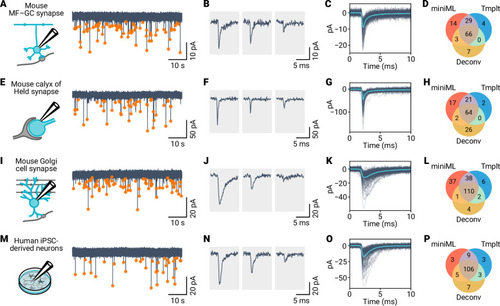
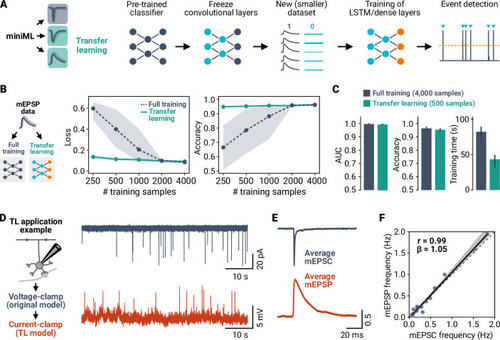
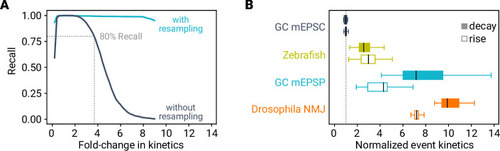
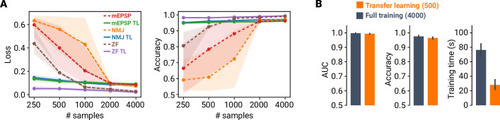
A deep learning framework for automated and generalized synaptic event analysis
- Authors
- O'Neill, P.S., Baccino-Calace, M., Rupprecht, P., Lee, S., Hao, Y.A., Lin, M.Z., Friedrich, R.W., Mueller, M., Delvendahl, I.
- Source
- Full text @ Elife
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |