- Title
-
LIPUS Promotes Calcium Oscillation and Enhances Calcium Dependent Autophagy of Chondrocytes to Alleviate Osteoarthritis
- Authors
- Guan, M., Han, X., Liao, B., Han, W., Chen, L., Zhang, B., Peng, X., Tian, Y., Xiao, G., Li, X., Kuang, L., Zhu, Y., Bai, D.
- Source
- Full text @ Adv Sci (Weinh)
|
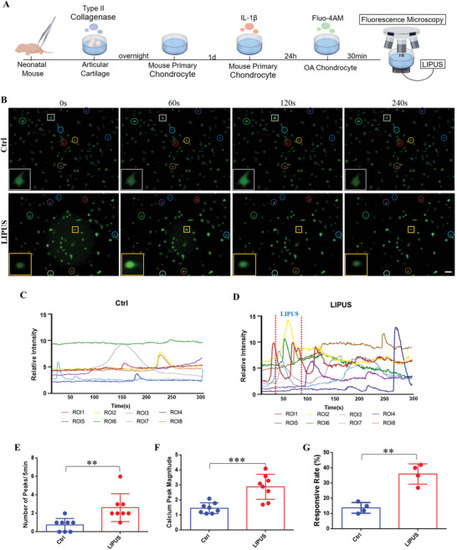
LIPUS activates Ca2+ signaling of inflammatory chondrocyte. A) Schematic of the inflammatory chondrocyte establishment and the real‐time calcium imaging with LIPUS. B) The real‐time fluorescence intensity images of chondrocyte at different time point in the same field of view (Circles of the same color represent the same cell, and square represent the typical cell in this field of view). Scale bar, 10 µm. Calcium transient relative fluorescence intensity of chondrocyte without C) and with D) LIPUS. Quantification of the number E) ( |
|
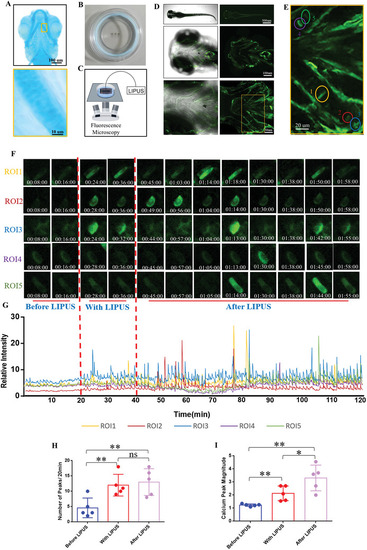
LIPUS promotes chondrocyte calcium oscillation of living zebrafish. A) Alcian blue staining to label the cranial cartilage structure of zebrafish juveniles. B) Zebrafish larvae were immobilized in a confocal dish with low‐melting‐point agarose. C) Schematic of the real‐time LIPUS‐processed calcium imaging under a confocal microscope. D) The whole fish, cranial and ceratohyal observed under a confocal microscope. E) 5 chondrocytes were circled with different colors as ROIs. F) The real‐time fluorescence intensity of the five ROIs. G) Calcium transient relative fluorescence intensity of ROIs. Quantification of the number H) ( |
|
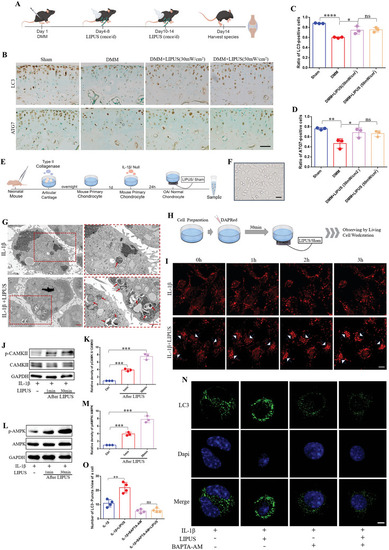
LIPUS up‐regulates chondrocyte autophagy in a calcium pathway‐dependent manner. A) Schematic of the mouse experiment procedures. B) Immunohistochemical staining of LC3 and ATG7 in the articular cartilage. Scale bar, 50 µm. Quantification of LC3‐positive cells C) and ATG7‐positive cells D) ( |
|
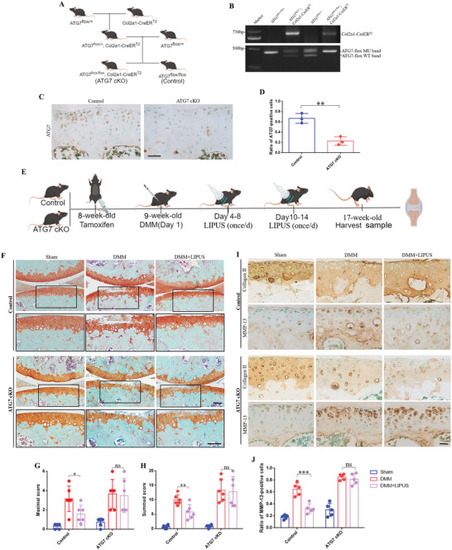
LIPUS maintains cartilage homeostasis depending on autophagy. A) Reproductive strategies for transgenic mice. B) Genotyping by PCR to confirm the Cre recombinase and homozygous floxed ATG7 in product. C)ATG7 deletion was confirmed by immunohistochemistry of the articular cartilage. Scale bar, 50 µm. D) Quantification of ATG7‐positive cells in the articular cartilage ( |
|
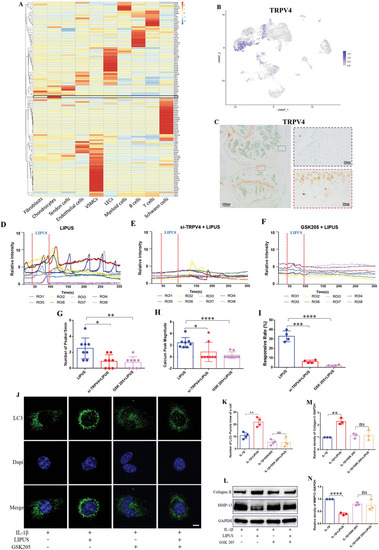
TRPV4 mediated Ca2+ signaling participates the regulation of LIPUS to inflammatory chondrocyte. A) Heatmap showing ion channel gene expression in all cells from the mouse ACLR model of PTOA. B) The express distribution of TRPV4 in UMAP plots. C) Immunohistochemical staining of TRPV4 in the mouse knee joint. Calcium transient relative fluorescence intensity of ROIs with LIPUS stimuli in control group D), in TRPV4‐knockdown group E), and in the TRPV4‐blocked group F). Quantification of the number G) ( |
|
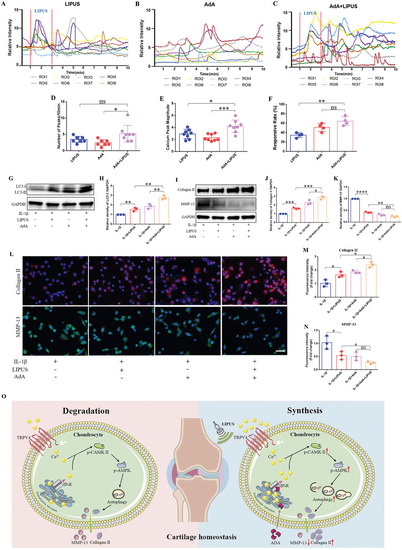
The intracellular calcium agonist AdA has a synergistic effect with LIPUS in regulating inflammatory chondrocyte. Calcium transient relative fluorescence intensity of ROIs in LIPUS alone A), in AdA alone B), in the combination of LIPUS and AdA C). Quantification of the number D) ( |