- Title
-
Identification, biological evaluation, and crystallographic analysis of coumestrol as a novel dual-specificity tyrosine-phosphorylation-regulated kinase 1A inhibitor
- Authors
- Peng, C.H., Hwang, T.L., Hung, S.C., Tu, H.J., Tseng, Y.T., Lin, T.E., Lee, C.C., Tseng, Y.C., Ko, C.Y., Yen, S.C., Hsu, K.C., Pan, S.L., HuangFu, W.C.
- Source
- Full text @ Int. J. Biol. Macromol.
|
Study workflow. We processed the compound library to eliminate PAINS. Subsequently, molecular docking of the compounds with DYRK1A was performed, and based on their docking scores and interactions with hinge residues, the identification process was initiated. After initial screening, a thorough visual assessment of the selected compounds' molecular conformation and practical applicability was carried out. Then, compounds were further screened with an enzyme-based assay, selectivity profiling, and in vitro testing. |
|
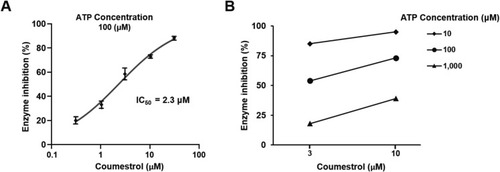
Coumestrol is an ATP-competitive DYRK1A inhibitor. An enzyme-based assay was used to (A) evaluate the inhibitory effect of coumestrol on DYRK1A. (B) Assessment of the effect of ATP concentration on coumestrol's inhibition of DYRK1A activity. The inhibition of DYRK1A by coumestrol was determined to be an ATP-competitive inhibition with an IC50 value of 2.3 ?M. |
|
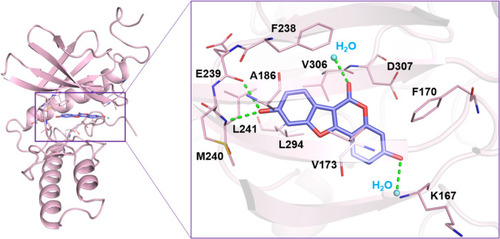
Coumestrol inhibits the activity of DYRK1A by occupying its ATP-binding site. Schematic showing x-ray crystallography of the interaction between DYRK1A and coumestrol, with coumestrol occupying the ATP-binding site of DYRK1A. |
|
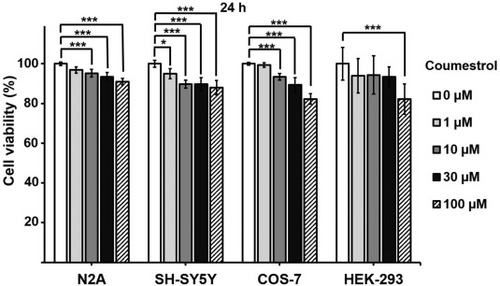
MTT assay for cytotoxicity of coumestrol. Neuronal cell lines (N2A and SH-SY5Y) and kidney cell lines (COS-7 and HEK-293) were treated with coumestrol. All results are presented as the mean ± SEM (n = 3). Statistical significance was determined by Kruskal-Wallis test followed by Dunn's post-hoc test (* p < 0.05, ** p < 0.01, *** p < 0.005). |
|
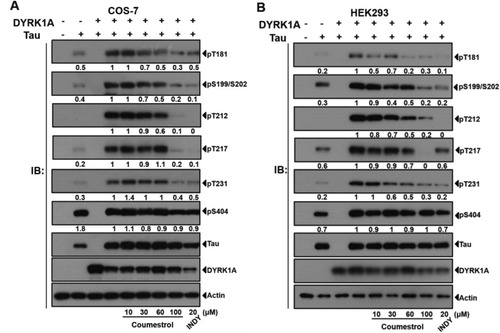
Coumestrol reduces phosphorylated (p)-tau levels in cells co-expressing DYRK1A and tau. (A) As indicated, COS-7 and (B) HEK293 cells were cotransfected with EGFP-Tau441, pDsRed-DYRK1A, or both. Cells were treated with coumestrol at the indicated concentrations. Detection of DYRK1A-dependent tau phosphorylation using phospho-specific antibodies to indicated sites. Quantifying band intensities in COS-7 and HEK293 cells showed a dose-dependent reduction of cellular tau phosphorylation after coumestrol treatment. |
|
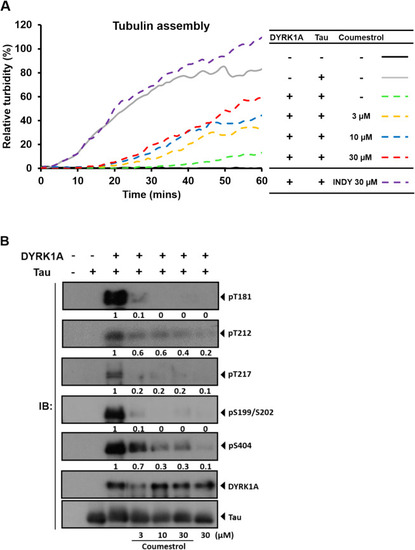
Coumestrol reduces tau phosphorylation levels and restores microtubule assembly in cell-free systems. (A) Turbidity increased over time (gray) when treated with the tau protein. Turbidity was reduced when co-treated with DYRK1A (green dashed line). Concomitant coumestrol treatment rescued the effect of DYRK1A treatment. (B) Phosphorylated (p)-tau levels were detected by Western blotting, and relative quantification was performed by normalizing p-tau to total tau. Coumestrol dose-dependently reduced p-tau levels. |
|
Neuroprotective effects of coumestrol against oligomeric amyloid-? (A?)-induced cytotoxicity. BV2 mouse microglia were pretreated with coumestrol and exposed to oligomeric A?. (A) After 24 h, BV2 cell viability was measured by MTT assay. (Coumestrol, n = 6; N-acetyl cysteine (NAC), n = 2) (B) Reactive oxygen species (ROS) levels were assessed by flow cytometry with H2DCFDA staining. Coumestrol pretreatment significantly restored BV2 cell viability and reduced ROS levels. All results are expressed as the mean ± SEM. Statistical significance was determined by Kruskal-Wallis test followed by Dunn's post-hoc test (* p < 0.05, ** p < 0.01, *** p < 0.005). |
|
Coumestrol mitigates neuronal damage caused by hyperphosphorylated tau in zebrafish larvae. (A) Representative images show motor neurite outgrowth in HuC:Kaede transgenic zebrafish larvae at 48 hpf in each group. Control group was microinjected with 50 pg of tau mRNA at 1-cell stage, and the other three experimental groups were microinjected with 50 pg of DYRK1A and 50 pg of tau mRNA. Between 20 and 48 hpf, experimental groups were immersed in 60 ?M of coumestrol, 0.08 ?M harmine or E3 medium as a vehicle control. Scale bar: 40 ?m. (B) Quantification of motor neurite outgrowth lengths (n = 18 per group) shows significantly reduced neurite outgrowth in the vehicle control group, but both coumestrol and harmine effectively protected the neurite outgrowth even with co-overexpression of DYRK1A and tau. (C) The successful rate of touch-evoked escape response in zebrafish larvae at 48 hpf (n = 30 per group) shows co-overexpression of DYRK1A and tau significantly compromised the escape reflex, but both coumestrol and harmine effectively rescued this phenotype. Data are presented as mean ± SEM. Statistical significance was determined by Kruskal-Wallis test followed by Dunn's post-hoc test (* p < 0.05, ** p < 0.01, *** p < 0.005). |
Reprinted from International journal of biological macromolecules, 282(Pt 4), Peng, C.H., Hwang, T.L., Hung, S.C., Tu, H.J., Tseng, Y.T., Lin, T.E., Lee, C.C., Tseng, Y.C., Ko, C.Y., Yen, S.C., Hsu, K.C., Pan, S.L., HuangFu, W.C., Identification, biological evaluation, and crystallographic analysis of coumestrol as a novel dual-specificity tyrosine-phosphorylation-regulated kinase 1A inhibitor, 136860, Copyright (2024) with permission from Elsevier. Full text @ Int. J. Biol. Macromol.