FIGURE
Fig. 6
- ID
- ZDB-FIG-250107-23
- Publication
- Peng et al., 2024 - Identification, biological evaluation, and crystallographic analysis of coumestrol as a novel dual-specificity tyrosine-phosphorylation-regulated kinase 1A inhibitor
- Other Figures
- All Figure Page
- Back to All Figure Page
Fig. 6
|
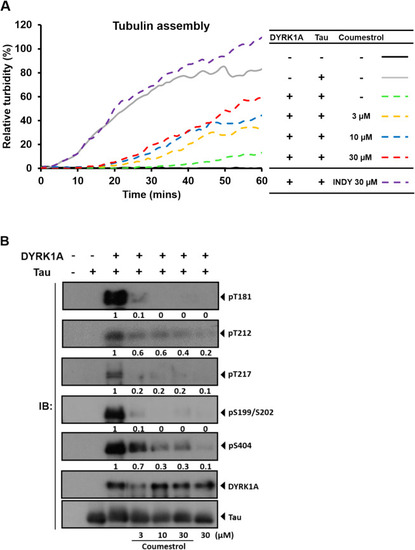
Coumestrol reduces tau phosphorylation levels and restores microtubule assembly in cell-free systems. (A) Turbidity increased over time (gray) when treated with the tau protein. Turbidity was reduced when co-treated with DYRK1A (green dashed line). Concomitant coumestrol treatment rescued the effect of DYRK1A treatment. (B) Phosphorylated (p)-tau levels were detected by Western blotting, and relative quantification was performed by normalizing p-tau to total tau. Coumestrol dose-dependently reduced p-tau levels. |
Expression Data
Expression Detail
Antibody Labeling
Phenotype Data
Phenotype Detail
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Reprinted from International journal of biological macromolecules, 282(Pt 4), Peng, C.H., Hwang, T.L., Hung, S.C., Tu, H.J., Tseng, Y.T., Lin, T.E., Lee, C.C., Tseng, Y.C., Ko, C.Y., Yen, S.C., Hsu, K.C., Pan, S.L., HuangFu, W.C., Identification, biological evaluation, and crystallographic analysis of coumestrol as a novel dual-specificity tyrosine-phosphorylation-regulated kinase 1A inhibitor, 136860, Copyright (2024) with permission from Elsevier. Full text @ Int. J. Biol. Macromol.