- Title
-
The zebrafish cerebellar neural circuits are involved in orienting behavior
- Authors
- Hosaka, S., Hosokawa, M., Hibi, M., Shimizu, T.
- Source
- Full text @ eNeuro
|
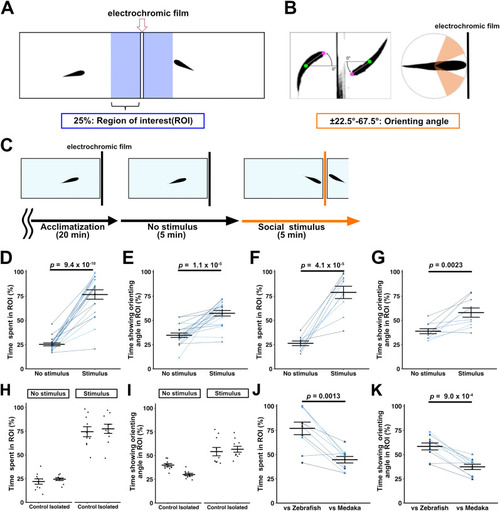
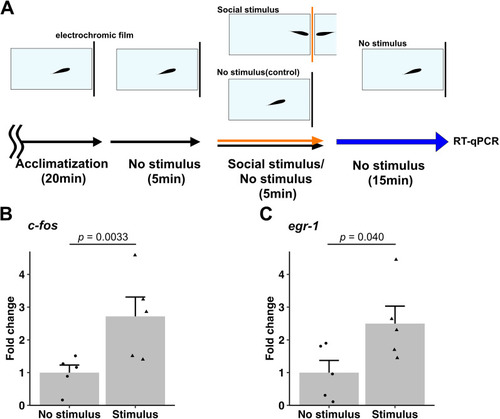
Orienting behavior of zebrafish toward conspecifics. |
|
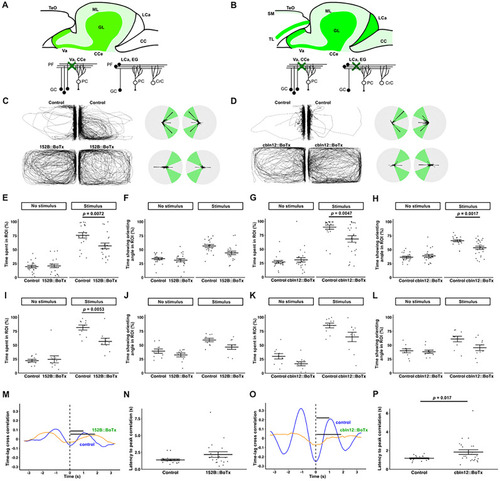
BoTx-mediated inhibition of GCs suppresses orienting behavior. Diagrams for the expression pattern of BoTx in adult transgenic zebrafish |
|
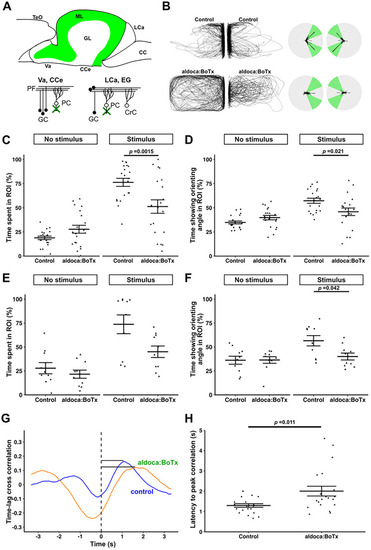
BoTx-mediated inhibition of PCs suppresses orienting behavior. Diagram for the expression pattern of BoTx in |
|
|
|
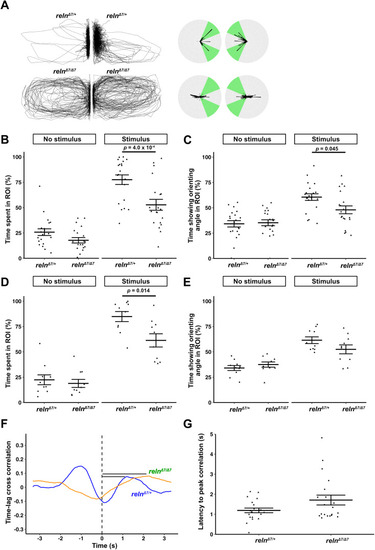
The cerebellum is activated during orienting behavior. |