- Title
-
Inflammation is a critical factor for successful regeneration of the adult zebrafish retina in response to diffuse light lesion
- Authors
- Bludau, O., Weber, A., Bosak, V., Kuscha, V., Dietrich, K., Hans, S., Brand, M.
- Source
- Full text @ Front Cell Dev Biol
|
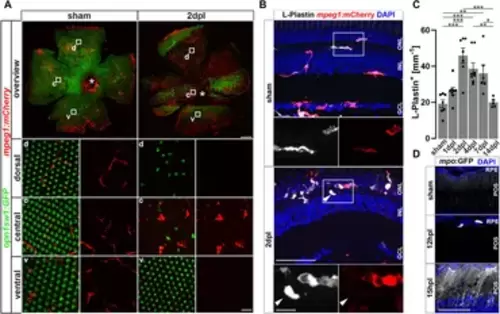
Sterile phototoxic ablation of photoreceptors triggers leukocyte accumulation. (A) Retinal flat mounts of Tg(opn1sw1:GFP) x Tg(mpeg1:mCherry) show the ramified structure of leukocytes in a UV-cone-specific reporter line and their reaction to lesion. Upon light lesion, leukocytes increase in number at the lesion site and display an amoeboid, activated morphology and accumulate in the central part of the lesion, while unharmed areas appear devoid of mpeg1:mCherry positive cells. A central stripe of autofluorescence can be seen (dashed lines) at 2 dpl. In the corresponding inset a few remaining UV-cones are visible. The optic nerve head is indicated by an asterisk. (B) Retinal sections show that the majority of L-Plastin+ cells are Tg(mpeg1:mCherry)+, display morphological changes after lesion, and accumulate at the outer nuclear layer (ONL) in response to lesion. However, L-Plastin+ Tg(mpeg1:mCherry) negative cells were also observed upon injury (arrowhead). (C) Quantification on sections of L-Plastin+ cells in sham and regenerating retinae at 1, 2, 4, 7 and 14 days post lesion (dpl) shows an increase of cells within 2 dpl that is resolved within 14 dpl. (D) In contrast to sham, Tg(mpo:GFP)+ neutrophils are detected upon lesion. They gather at the lesion site (12 hpl) and a diffuse GFP positive pattern (matrix) is formed from 15hpl onwards. Scale bars in A: overview 200 μm, Insets 10 µm; Scale Bars in D: overview 50µm, insets 10µm; error bars indicate SEM; * = p ≤ 0.05; ** = p ≤ 0.01; *** = p < 0.001; N ≥ 6; one-way ANOVA Tukey’s post hoc analysis; ONL = outer nuclear layer, INL = inner nuclear layer; GCL = ganglion cell layer; POS = photoreceptor outer segments; RPE = retina pigment epithelium; d = dorsal; c = central; v = ventral. |
|
Müller glia transiently activate the NF-κB:GFP reporter and express the NF-κB target metalloproteinase mmp9 in response to injury. (A) In homeostatic retina the NF-κB:GFP reporter is expressed in the vasculature residing below the ganglion cell layer (asterisk) and in microglial cells (arrowheads). In comparison to sham, the NF-κB:GFP reporter is activated in response to lesion in gfap:NLS-mCherry labeled Müller glia, and in photoreceptors at the outer nuclear layer (ONL), and is predominantly active in Müller cells at 4 dpl. (B) In situ hybridization of mmp9, in combination with immunohistochemistry for proliferating cell nuclear antigen (PCNA) and glial fibrillary acidic protein (GFAP/Zrf-1) labeling Müller glia, shows that mmp9 is not expressed in sham retinae. In contrast, mmp9 is transiently expressed at one and 2 dpl and returns to undetectable levels at 4 dpl. Scale bar: 50 µm, insets 10 µm. INL = inner nuclear layer; GCL = ganglion cell layer. |
|
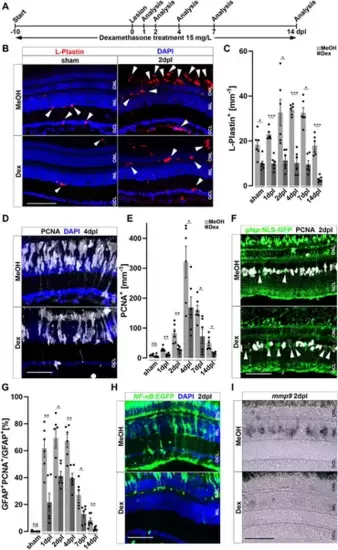
Immunosuppression interferes with leukocyte accumulation and Müller glia reactivity. (A) Scheme of experimental outline. Fish were treated with Dexamethasone (Dex) or vehicle (Methanol; MeOH) from 10 days prior to injury until the day of analysis (sham, 1, 2, 4, 7 and 14 days post lesion; dpl). (B) Dex-treatment reduces the number of L-Plastin+ leukocytes (arrowheads) in sham (left panel) and regenerating retinae at 2 dpl (right panel). (C) Quantification of L-Plastin + cells in MeOH- and Dex-treated sham or lesioned animals at 1, 2, 4, 7 and 14 dpl. (D) Immunohistochemistry for proliferating cell nuclear antigen (PCNA) reveals impaired proliferation in Dex-treated animals at 4 dpl. (E) Quantification of PCNA + cells in MeOH- and Dex-treated sham and regenerating retinae at 1, 2, 4, 7 and 14 dpl indicates impaired proliferative response in the Dex-treated group. (F) Immunohistochemistry for PCNA in gfap:NLS-GFP labeled Müller glia shows reduced numbers of proliferating Müller glia in the Dex treated group at 2 dpl compared to MeOH controls (arrowheads indicating non proliferative Müller cells). (G) Quantification of proliferating Müller glia in vehicle and Dex-treated sham and regenerating retinae at 1, 2, 4, 7, and 14 dpl, indicating that Dex hinders Müller glia proliferation. (H) In comparison to vehicle, the NFκB:GFP reporter is not activated in the inner nuclear layer (INL) of Dex-treated retinae at 2 dpl. (I) Injury-induced expression of mmp9 is strongly reduced in Dex-treated retinae at 2 dpl. Retinal layering is indicated by dashed lines. Scale bar: 50 μm, Error bars indicate SEM; ns = p > 0.05, * = p ≤ 0.05; ** = p ≤ 0.01; *** = p < 0.001; N = 6; two-tailed t-Test, ONL = outer nuclear layer, INL = inner nuclear layer GCL = ganglion cell layer. |
|
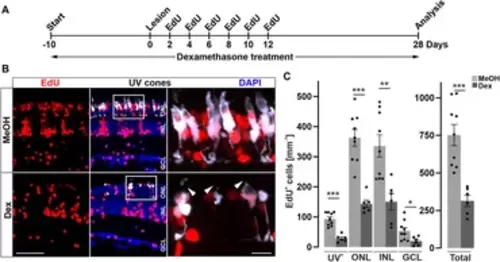
Long-term immune suppression impairs photoreceptor regeneration. (A) Scheme of experimental outline. opn1sw1:GFP transgenic animals (labeling UV-cones) were treated with Dexamethasone (Dex) or vehicle (Methanol; MeOH) from 10 days prior to lesion until the day of analysis (28 days post lesion; dpl; 38 days post treatment; dpt). EdU pulses were applied at 2, 4, 6, 8, 10, and 12 dpl. (B) In comparison to MeOH-treated animals, the number of regenerated EdU+ UV-cones is significantly reduced after Dex-treatment (compare upper panel with lower panel). Furthermore, the morphology of photoreceptor cells is disrupted in comparison to MeOH controls (arrowheads). (C) Quantification of EdU+ cells with respect to retinal layers show significant reduction of EdU positive nuclei in the Dex-treated group. Scale bars: 50µm, insets 10 µm. Error bars indicate SEM; * = p ≤ 0.05; ** = p ≤ 0.01; *** = p < 0.001; N ≥ 6; two-tailed t-Test. ONL = outer nuclear layer; INL = inner nuclear layer; GCL = ganglion cell layer. |
|
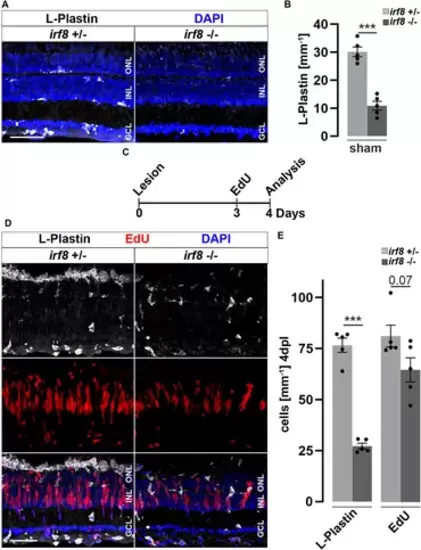
Genetic reduction of microglia impairs retinal reactive proliferation in response to injury. (A) In contrast to heterozygous control siblings (irf8+/−), interferon regulatory factor 8 (irf8) homozygous myeloid-deficient mutant retinae (irf8−/−) display a decreased number of L-Plastin+ leukocytes in homeostasis. (B) Quantifications of L-Plastin+ cells in irf8−/− and control heterozygous siblings irf8+/−. (C) Scheme of experimental outline. irf8−/− and irf8+/− animals received an EdU pulse at 3 days post lesion (dpl) and were analyzed at 4 dpl. (D) During regeneration, the accumulation of L-Plastin+ cells at the outer nuclear layer (ONL) is impaired in irf8−/− but not in control irf8+/− siblings. Moreover, the number of EdU+ cells appears decreased in irf8−/− retinae. (E) Quantification of L-Plastin+ cells and EdU+ nuclei in irf8−/− and control irf8+/− animals at 4 dpl, showing reduced amounts of positive cells, respectively. Scale bar: 50 µm. Error bars indicate SEM; *** = p < 0.001; N = 5; two-tailed t-Test; INL = inner nuclear layer; GCL ganglion cell layer. |
|
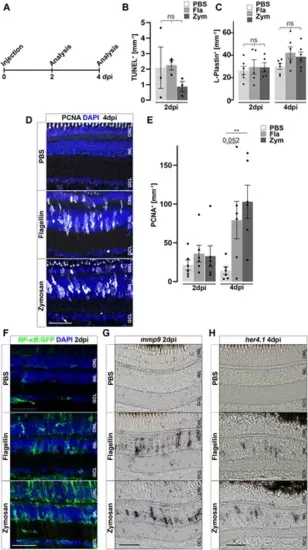
Inflammatory stimuli trigger Müller glia reactivity. (A) Scheme of experimental outline. Wild type or NF-κB:GFP reporter animals were injected with flagellin or zymosan into the vitreous of the eye and analyzed at 2 and 4 days post injection (dpi). (B) Quantification of TUNEL+ nuclei in control and injected retinae at two dpi revealing no increase in cell death. See also Supplementary Figure S7 for counts of pyknotic nuclei. (C) Quantification of L-Plastin+ cells in control (PBS) and flagellin or zymosan injected retinae at two and four dpi display no significant increase in leukocytes due to the injection of factors. (D) Immunohistochemistry for proliferating cell nuclear antigen (PCNA) reveals proliferation in flagellin or zymosan injected but not in control animals at four dpi. Signal in the outer nuclear layer (ONL) appears to be a non-specific labeling of photoreceptor cells. (E) Quantification of PCNA+ nuclei in injected retinae at two and four dpi. (F) In comparison to PBS injected animals, injection of flagellin or zymosan results in strong activation of the NFκB:GFP reporter at two dpi. (G & H) In situ hybridization of mmp9 and her4.1 show expression of both genes in flagellin or zymosan injected, but not in PBS injected, eyes at two and four dpi, respectively. Retinal layering is indicated by dashed lines. Scale bar: 50 μm. Error bars indicate standard error; * * = p≤ 0.01 B: N = 3 Fish; C&E: N = 6 Fish; One-tailed ANOVA; ONL = outer nuclear layer; INL = inner nuclear layer; GCL = ganglion cell layer. |
|
M-CSF stimulates an inflammatory response and initiates Müller glia cell cycle re-entry in the absence of a lesion. (A) Scheme of experimental outline. Wild type or NFκB:GFP reporter animals were injected with M-CSF or PBS into the vitreous of the eye and analyzed at 2 and 4 days post injection (dpi). (B) Quantification of TUNEL+ nuclei in control and M-CSF-injected retinae at two dpi reveals no chance of cell death. See also Supplementary Figure S7 for counts of pyknotic nuclei. (C) Immunohistochemistry for proliferating cell nuclear antigen (PCNA) reveals proliferation in close proximity to Zrf-1 (GFAP) positive Müller cells in M-CSF injected but not in control injected animals at four dpi. n both conditions a slight nonspecific labeling can be seen in the ONL. (D) Quantification of PCNA+ cells in control (PBS) and M-CSF injected retinae at four dpi. (E) L-Plastin staining shows more positive cells in M-CSF injected retinae at four dpi compared to controls. The location in the inner nuclear layer (INL) and morphology of the leukocytes indicates reactivity. (F) Quantification of L-Plastin+ cells in control and M-CSF injected retinae at four dpi, show accumulation of L-Plastin cells in M-CSF injected specimen. (G) In comparison to PBS, injection of M-CSF results in strong activation of the NFκB:GFP reporter at two dpi. (H) In situ hybridization of mmp9 shows transcriptional activation of this gene in M-CSF injected but not in control-injected animals at two dpi. Scale bars = 50µm, Error bars indicate SEM; ns > 0.05; ** = p ≤ 0.01; *** = p < 0.001; N ≥ 5; two-tailed Student’s t-test. ONL = outer nuclear layer; GCL = ganglion cell layer. |
|
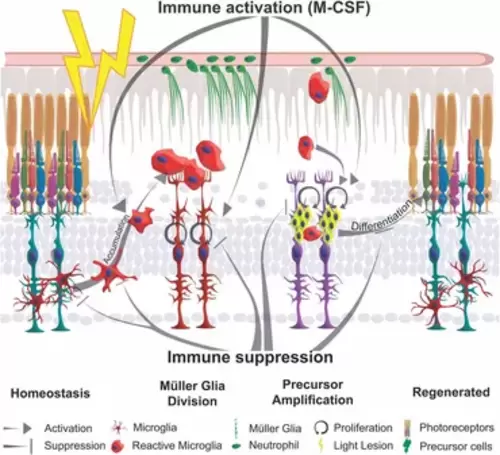
Schematic summary of the impact of inflammation in retinal regeneration. In response to injury, microglia undergo a phenotypic change from ramified to amoeboid shape and accumulate at the lesion site. This process is inhibited by immune suppression. The reactive proliferation of Müller glia during regeneration is also impaired upon immunosuppression. Leukocytes stimulate the proliferation of neuronal precursor cells. M-CSF as inflammatory mediator triggers Müller glia proliferation, leukocyte accumulation and the generation of neuronal precursor cells. This process may also act indirectly via the stimulation of immune cells. Overall, the innate immune system, via mediators such as M-CSF, supports the differentiation into photoreceptor cells and thereby acts as a regenerative cue for retina regeneration. |