- Title
-
Inhibition of PIKfyve Leads to Lysosomal Disorders via Dysregulation of mTOR Signaling
- Authors
- Xia, J., Wang, H., Zhong, Z., Jiang, J.
- Source
- Full text @ Cells
|
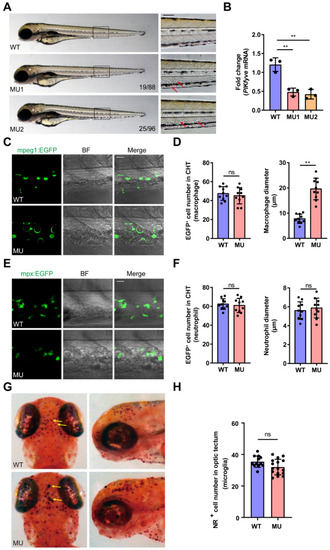
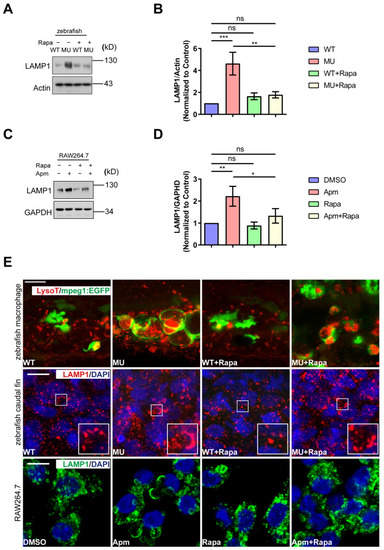
Defects in macrophages in PIKfyve mutant zebrafish embryos. ( PHENOTYPE:
|
|
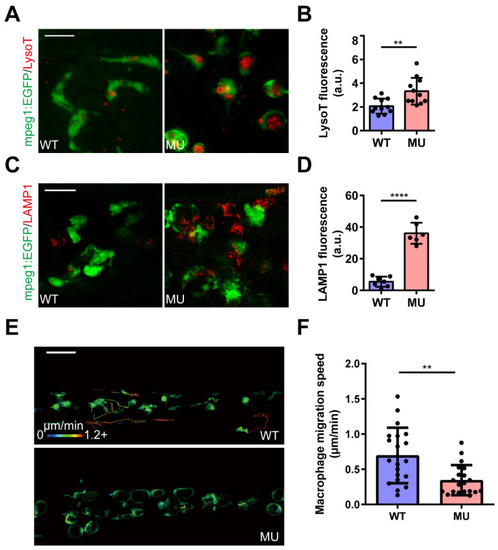
PIKfyve deficiency leads to the accumulation of enlarged LAMP1-positive compartments in macrophages. ( PHENOTYPE:
|
|
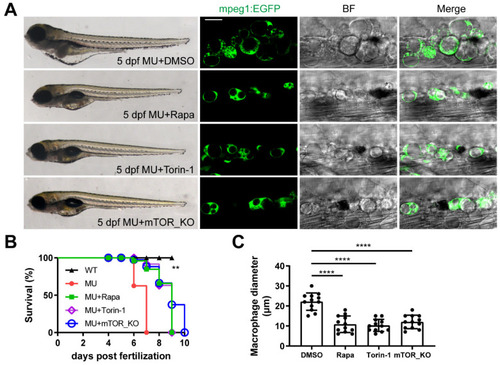
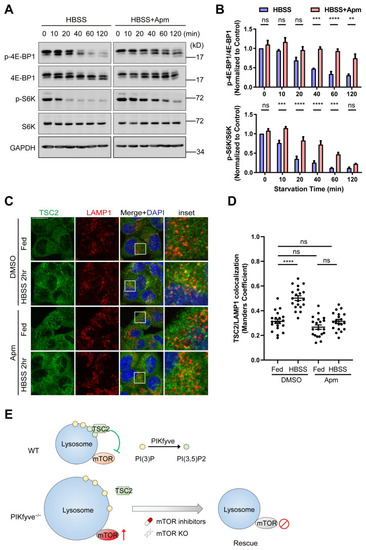
The defects in PIKfyve mutants are dependent on sustained mTOR activation. ( |
|
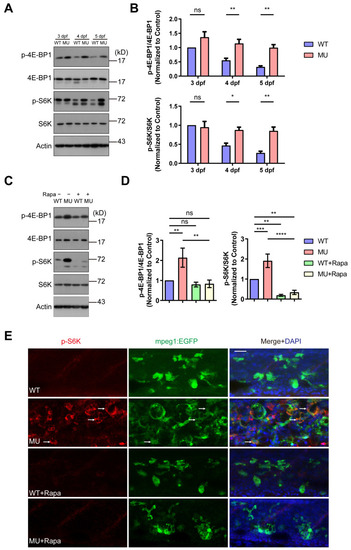
Molecular evidence for the hyperactivation of mTOR signaling in PIKfyve mutant zebrafish. ( PHENOTYPE:
|
|
Rapamycin treatment ameliorates the lysosomal defects induced by PIKfyve deficiency in vivo and in vitro. ( PHENOTYPE:
|
|
PIKfyve is required for starvation-induced mTOR shutdown. ( |