- Title
-
Zebrafish Avatar-test forecasts clinical response to chemotherapy in patients with colorectal cancer
- Authors
- Costa, B., Estrada, M.F., Gomes, A., Fernandez, L.M., Azevedo, J.M., Póvoa, V., Fontes, M., Alves, A., Galzerano, A., Castillo-Martin, M., Herrando, I., Brandão, S., Carneiro, C., Nunes, V., Carvalho, C., Parvaiz, A., Marreiros, A., Fior, R.
- Source
- Full text @ Nat. Commun.
|
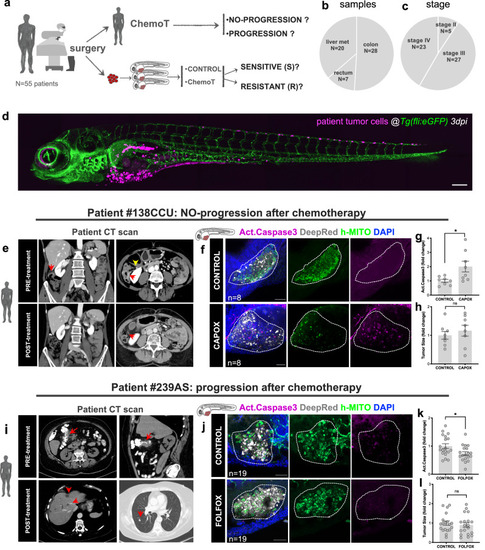
Clinical study design, patient cohort and examples of zAvatars analysis. |
|
Apoptosis in CRC zAvatars predicts patient clinical response to treatment. |
|
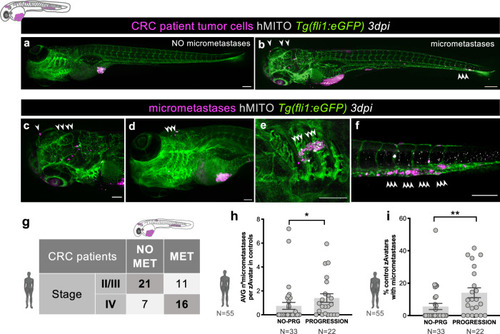
Metastatic potential in zAvatars correlates with tumor staging and patient clinical progression. |
|
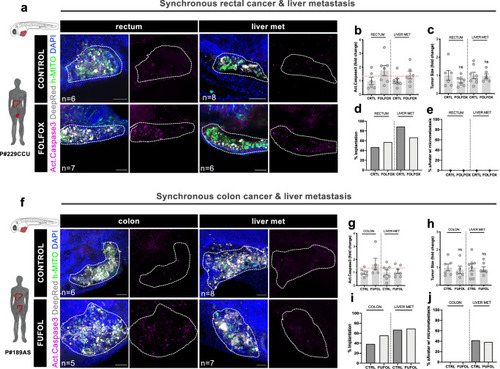
zAvatars can reveal patient intra-tumoral heterogeneity. |
|
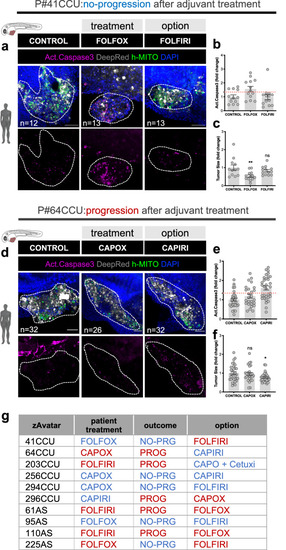
The zAvatar-test can be used to test alternative therapy options. |
|
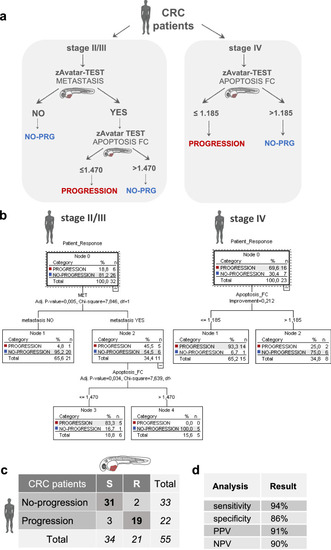
Decision tree model improves accuracy of the zAvatar-test. |
|
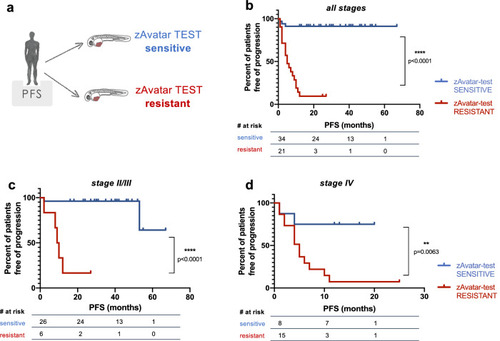
Patients with a sensitive zAvatar-test have longer Progression-Free Survival. |
|
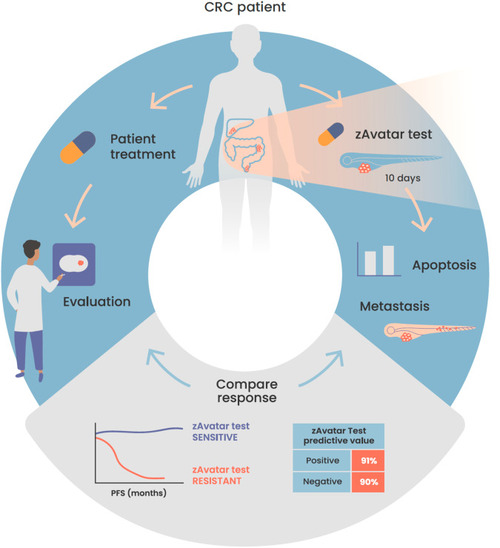
Schematic illustration of the workflow of the zAvatar-test and obtained results. Our findings demonstrate that the zAvatar-test is an accurate screening-platform for predicting colorectal cancer treatment outcomes. Illustration by Marta Correia. |