- Title
-
PAX1 represses canonical Wnt signaling pathway and plays dual roles during endoderm differentiation
- Authors
- Miao, D., Ren, J., Jia, Y., Jia, Y., Li, Y., Huang, H., Gao, R.
- Source
- Full text @ Cell Commun. Signal.
|
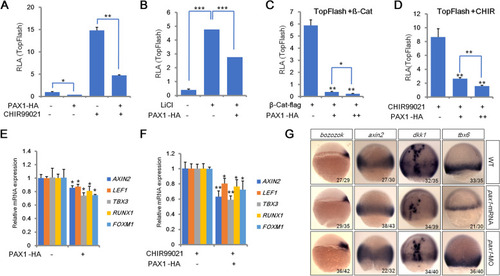
PAX1 plays a negative role in canonical Wnt signaling pathway. |
|
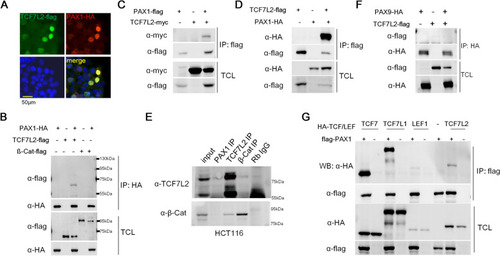
PAX1 interacts with TCF7L2. |
|
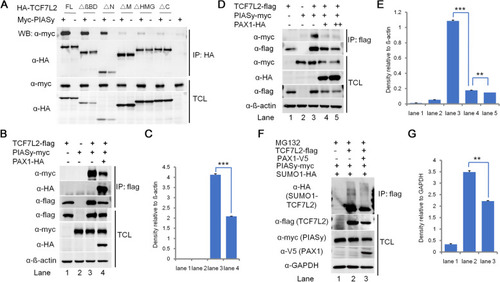
PAX1 inhibits the interaction between TCF7L2 and PIASy, and decreases the SUMOylation level of TCF7L2. |
|
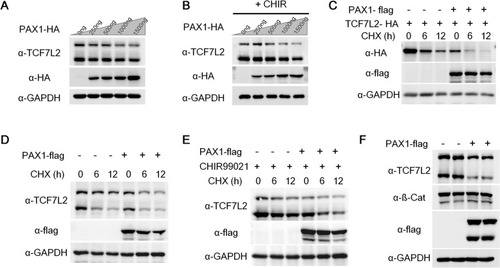
PAX1 reduces TCF7L2 protein stability. |
|
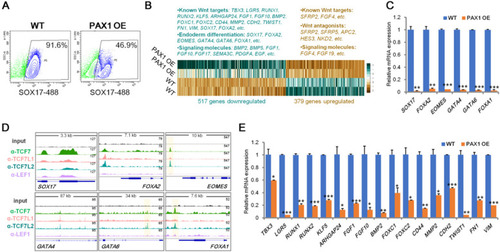
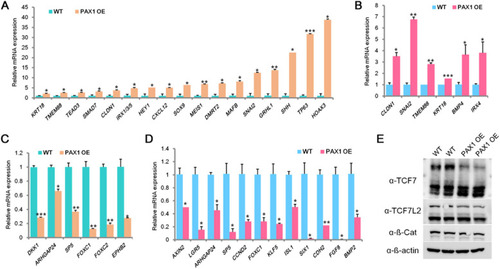
PAX1 ectopic expression leads to impaired definitive endoderm differentiation by inhibiting Wnt signaling. |
|
PAX1 represses Wnt signaling pathway in foregut and pharyngeal endoderm cells. |
|
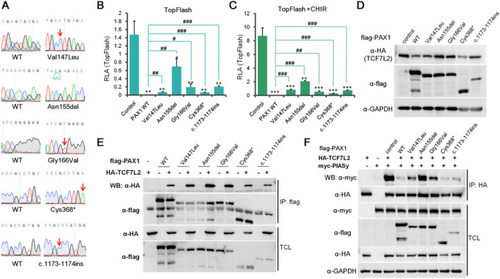
The roles of PAX1 mutants associated with SCID in regulation of Wnt signaling pathway. |