- Title
-
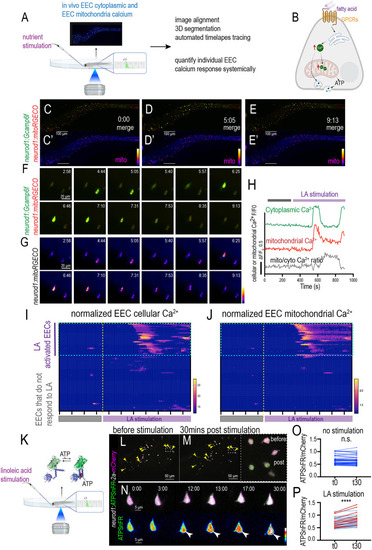
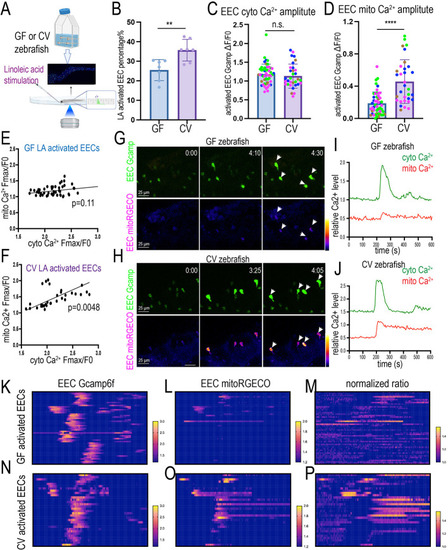
Gut microbiota regulates the nutrient sensing enteroendocrine cell maturation and mitochondrial function
- Authors
- Alsudayri, A., Perelman, S., Brewer, M., Chura, A., McDevitt, M., Drerup, C., Ye, L.
- Source
- Full text @ Development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|