- Title
-
Color-Flu Fluorescent Reporter Influenza A Viruses Allow for In Vivo Studies of Innate Immune Function in Zebrafish
- Authors
- Soos, B.L., Ballinger, A., Weinstein, M., Foreman, H., Grampone, J., Weafer, S., Aylesworth, C., King, B.L.
- Source
- Full text @ Viruses
|
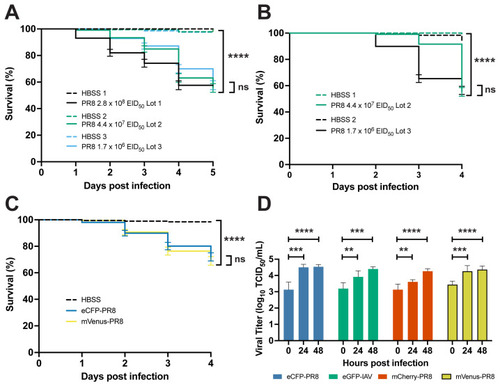
Characterization of PR8 and Color-flu systemically infected AB zebrafish. ( |
|
Characterization of PR8 and Color-flu systemic infection across zebrafish lines. ( |
|
Confocal imaging of Color-flu-infected zebrafish. ( |
|
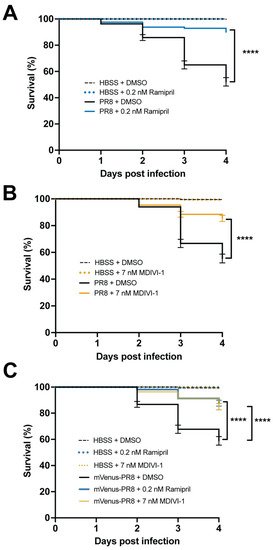
Ramipril and MDIVI-1 increase survival in systemically infected PR8 and Color-flu-infected 3 dpf zebrafish. ( |
|
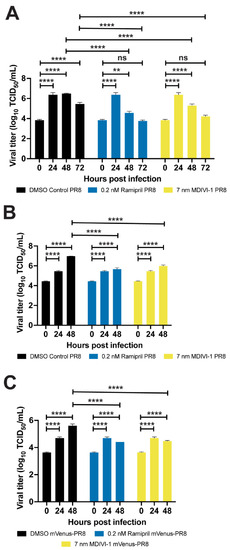
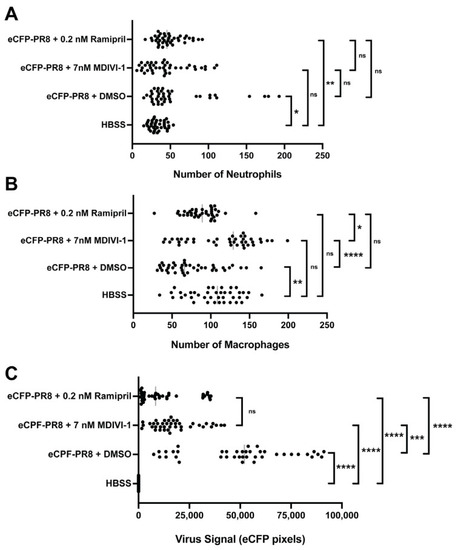
Ramipril and MDIVI-1 treatments lower viral burden in IAV-infected zebrafish. ( |
|
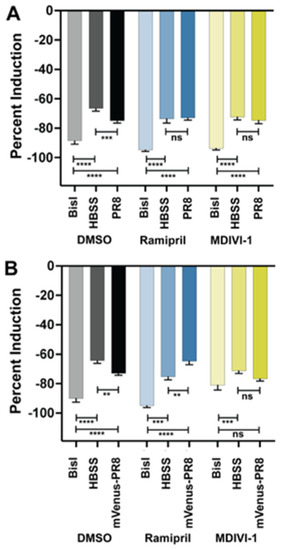
Ramipril and MDIVI-1 treatment alters the respiratory burst response in IAV systemically infected zebrafish at 48 hpi. ( |
|
Quantification of the number of neutrophils ( |