- Title
-
Extracellular Matrix Disorganization Caused by ADAMTS16 Deficiency Leads to Bicuspid Aortic Valve With Raphe Formation
- Authors
- Lin, Y., Yang, Q., Lin, X., Liu, X., Qian, Y., Xu, D., Cao, N., Han, X., Zhu, Y., Hu, W., He, X., Yu, Z., Kong, X., Zhu, L., Zhong, Z., Liu, K., Zhou, B., Wang, Y., Peng, J., Zhu, W., Wang, J.
- Source
- Full text @ Circulation
|
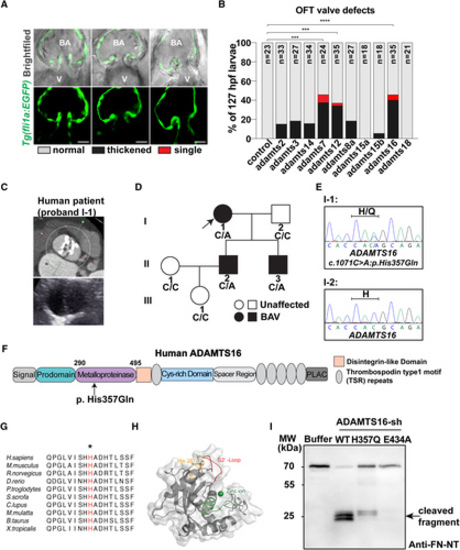
ADAMTS16 was identified as a candidate gene involved in aortic valve abnormalities. A, Representative images different phenotypes observed in the 10 family adamts gene-targeted larval zebrafish morphants, showing either thickened (middle column, 1 image selected from 1 adamts16 morphant) or single (right column selected from one adamts7 morphant) OFT valves compared with normal OFT valves (left column from one control morphant) at 127 hpf. Scale bars=20 µm. B, Percentage occurrence of each OFT valve phenotype (normal valve in blank, thickened valve in black or single valve in red) observed in 127 hpf zebrafish larvae from each adamts gene morphant (the numbers analyzed are as follows: control=23; adamts2=33; adamts3=27; adamts14=34; adamts7=24; adamts12=35; adamts8a=27; adamts15a=18; adamts15b=18; adamts16=35; and adamts18=21). P values were calculated using the Yates χ2 or χ2 test or Fisher exact test as appropriate. ****P<0.0001; ***P<0.001. Zebrafish larvae with severe pericardial edema were excluded to minimize the risk of secondary phenotypes. C, Contrast computed tomography of the proband (I-1) indicated type 0 BAV, severe calcification, and moderately dilated ascending aorta with a diameter of 40 mm. D, Pedigree and genotype of each family member of the proband (I-1). Arrow indicates the proband; squares indicate male family members; circles indicate female members; black fillings indicate family members diagnosed with BAV; diagonal lines indicate deceased family members. E, Heterozygous missense variant ADAMTS16 c.1071C>A was validated through Sanger sequencing. F, Schematic diagram of the ADAMTS16 protein domains showing the location of ADAMTS16 p. H357Q variant. G, The affected histidine (H, in red highlighted by an asterisk) in ADAMTS16 is within a highly conserved region across various species. H, Predicted protein structure of the metalloproteinase domain of ADAMTS16 modeled by AlphaFold2: catalytic site and zinc ion (green), S2’-loop (red), and affected amino acid (orange). I, Western blot analysis of the fibronectin N-terminal 70-kDa peptide incubated with recombinant ADAMTS16-sh protein (WT, H357Q, E434A) shows fewer 30-kDa fibronectin fragments released by ADAMTS16 H357Q compared with ADAMTS16 WT (black arrow). A indicates alanine; ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; BAV, bicuspid aortic valve; E, glutamate; FN, fibronectin; Gln, glutamine; H, histidine; hpf, hours postfertilization; MW, molecular weight; NT, N-terminal; OFT, outflow tract; Q, glutamine; and WT, wild-type. PHENOTYPE:
|
|
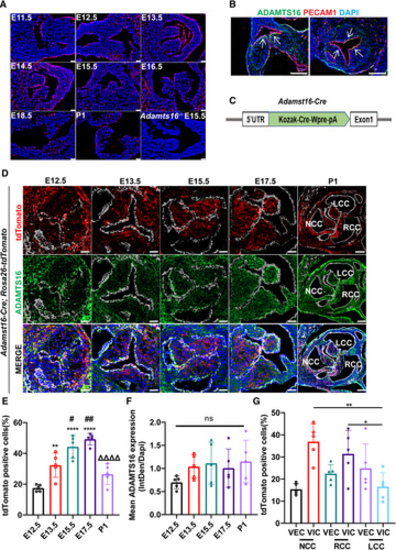
Spatiotemporal expression pattern of Adamts16 during aortic valve development in mice. A, Fluorescence in situ hybridization quantification of Adamts16 mRNA expression (red) at different stages of aortic valve development (between embryonic E10.5 and E18.5 and at P0). Scale bars=25 µm. B, Immunostaining of ADAMTS16 protein (green) and PECAM1 (red) at E14.5 in both transversal and sagittal sections. The white arrow indicates subendothelial localization of the ADAMTS16 protein. Scale bars=100 µm. C, Schematic strategy for the generation of the Adamts16-Cre knockin mouse line. D, Adamts16-Cre;Rosa26-tdTomato lineage tracing mice were generated by cross-breeding the Adamts16-Cre line with the Rosa26-tdTomato reporter line. Immunostaining of ADAMTS16 (green), PECAM1 (white), and red fluorescent protein in aortic valve at E12.5, E13.5, E15.5, E17.5, and neonatal P1. Scale bars=100 µm. E, Quantification of total tdTomato-positive cells during aortic valve development. Data are presented as the mean±SD (n=5). A P value of <0.05 was considered significant. The asterisk indicates the comparisons between the E12.5 time point with each of the other 4 time points, the hash indicates the comparison between the E13.5 time point with each other 3 time points, and the triangle indicates comparison between the E17.5 time point and the neonatal time points. F, Quantification of ADAMTS16 staining during aortic valve development. Data are presented as the mean±SD (n=5). A P value of <0.05 was considered significant. E and F, Two-way ANOVA was performed for multiple-group comparisons followed by the Tukey multiple-comparisons test. G, Quantification of tdTomato-positive cells in the 3 valve cusps at P1. Data are presented as the mean±SD (n=5). P values were calculated using 2-way ANOVA with the Tukey multiple comparisons test. **P<0.01; *P<0.05. ADAMTS indicates a disintegrin and metalloproteinase with thrombospondin motifs; E, embryonic day; P1, postnatal day 1; NCC, noncoronary cusp; LCC, left coronary cusp; RCC, right coronary cusp. |
|
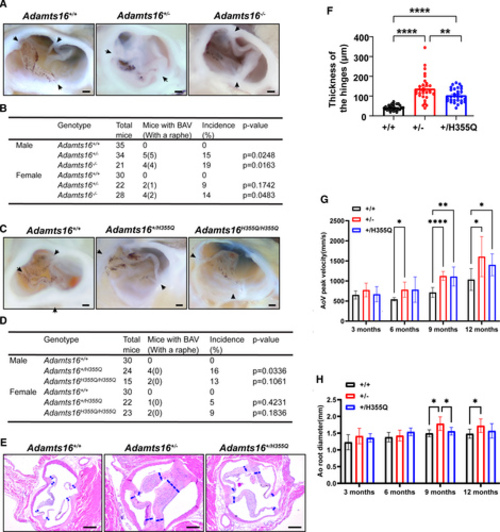
Phenotypes of both Adamts16+/- and Adamts16+/H355Q mice showing the occurrence of BAV and progressive aortic valve disease. A, Anatomic analysis of aortic valves in Adamts16+/+, Adamts16+/-, and Adamts16-/- mice. The black arrow indicates commissures. Scale bars=1 mm. B, Percentage of bicuspid aortic valve occurrence in Adamts16+/+, Adamts16+/-, and Adamts16-/- mice. P values were calculated using the Fisher exact test . C, Anatomic analysis of aortic valves in Adamts16+/+, Adamts16+/H355Q, and Adamts16H355Q/H355Q mice. The black arrow indicates commissures. Scale bars=1 mm. D, Percentage of bicuspid aortic valve occurrence in Adamts16+/+, Adamts16+/H355Q, and Adamts16H355Q/H355Q mice. P values were calculated using the Fisher exact test. All mice used for aortic valve anatomic analysis were at 3 to 12 months. E, Representative hematoxylin and eosin–stained aortic valve images in mouse transverse sections at 12 months of age. The blue dashed lines represent regions of quantification for thickness. Scale bars=200 µm. F, Quantification of aortic valve thickness reveals significant enlargement of the aortic valve leaflet hinges in Adamts16+/+, Adamts16+/-, and Adamts16+/H355Q mice (n=5 mice for each genotype; each mouse has 6 valve hinge measurements). ****P<0.0001, mean±SEM, using 1-way ANOVA followed by the Tukey post hoc test. G, Quantification of aortic valve peak velocities. Aortic valve peak velocities were significantly increased at 9 and 12 months in Adamts16+/- and Adamts16+/H355Q mice, whereas no increase was observed in Adamts16+/+ control littermates (n=8 mice for each genotype at each stage, mean±SEM, using mixed-effects ANOVA followed by the Tukey post hoc test). H, Quantification of aortic root diameter. Aortic root diameter progressively increased in 9- and 12-month-old Adamts16+/- mice, whereas no obvious increase was observed in Adamts16+/H355Q mice and Adamts16+/+ control littermates (n=8 mice for each genotype at each stage, mean±SEM, using mixed-effects ANOVA followed by the Tukey post hoc test). ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05. ADAMTS indicates a disintegrin and metalloproteinase with thrombospondin motifs; Ao root, aortic root; AoV, aortic valve; and BAV, bicuspid aortic valve. |
|
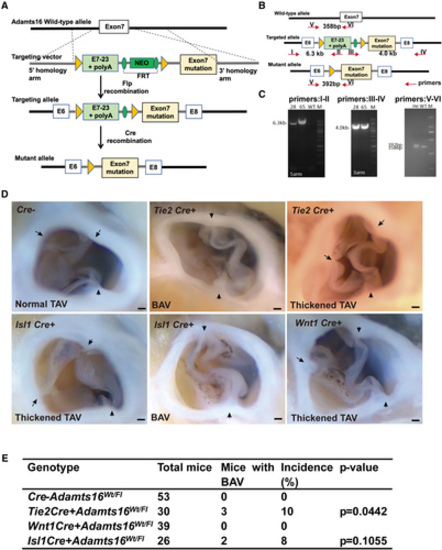
Endothelial cell– and secondary heart field cell–specific Adamts16 H355Q mutations recapitulate the BAV phenotype. A, Schematic strategy for the generation of the conditional Adamts16 H355Q mutant knockin mouse line by Cre recombination. B, Schematic showing the location of primers used for reverse transcription-polymerase chain reaction (RT-PCR) analysis of the conditional Adamts16 H355Q mutant knockin mouse line; predicted band sizes are indicated. C, RT-PCR analysis process based on the primer pairs designed as described above for identifying Adamts16+/flox-H355Q;Cre+ mice by detecting a specific 392-bp band of the amplicon of the polymerase chain reaction. The numbers 28 and 65 indicate mouse identity documents, both of which were Adamts16+/flox-H355Q;Cre+ mice according to the RT-PCR result compared with that of a WT mouse. Note that the polymerase chain reaction result for identifying the Cre recombinase is not shown. D, Anatomic analysis of aortic valves in Adamts16+/flox-H355Q;Tie2-Cre+, Adamts16+/flox-H355Q;Wnt1-Cre+, Adamts16+/flox-H355Q;Isl1-Cre+, and Adamts16+/flox-H355Q mice at 3 months of age. The black arrow indicates commissures. Scale bars=1 mm. E, The BAV incidences of Adamts16+/flox-H355Q;Tie2-Cre+ (3 of 30), Adamts16+/flox-H355Q;Wnt1-Cre+ (0 of 39), Adamts16+/flox-H355Q;Isl1-Cre+ (2 oof 26), and Adamts16+/flox-H355Q (0 of 53) mice. P values were calculated using the Fisher exact test. ADAMTS indicates a disintegrin and metalloproteinase with thrombospondin motifs; BAV, bicuspid aortic valve; He, heterozygous; M, marker; and WT, wild-type. |
|
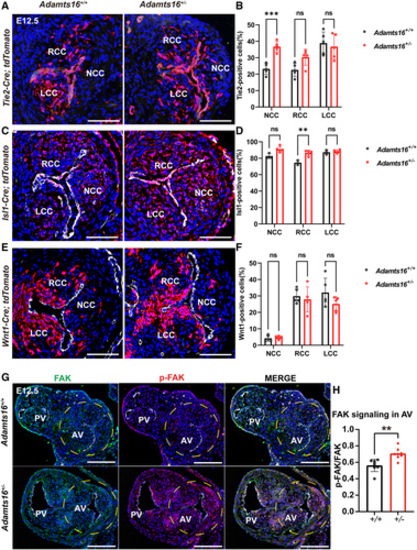
The effects of Adamts16 deficiency on the activities of each cell lineage during the development of the aortic valve. A through F, Fate mapping of the endothelial ( A), neural crest ( C), and second heart field ( E) lineage cells was performed respectively in the aortic valves of both Adamts16+/+ and Adamts16+/- embryos at E12.5. Transversal sections were immunoassayed with PECAM1 (in white) and DAPI (in blue). Scale bars=100 µm. Quantification of endothelial-derived cells demonstrates the increased cell numbers in the noncoronary cusp in Adamts16+/- (n=5) compared with Adamts16+/+ littermates (n=5; **P<0.01; B). Quantification of second heart field–derived cells demonstrates increased cell numbers in the noncoronary and right coronary cusps in Adamts16+/- mice (n=5) compared with Adamts16+/+ littermates (n=3). **P<0.01; ****P<0.0001 ( D). Quantification of neural crest–derived cells demonstrates that there was no significant difference in cell number in 3 individual cups of Adamts16+/- (n=5) compared with Adamts16+/+ littermates (n=5) ( F). In B, D, and F, Statistical analysis was performed using 2-way ANOVA followed by the Šidák multiple comparisons test. G, Immunostaining of FAK and phospho-FAK (Tyr397) in aortic valves of Adamts16+/+ and Adamts16+/- embryos at E12.5. Scale bars=100 µm. H, Quantification of phospho-FAK (Tyr397) staining (n=8 for each genotype). P values were calculated using the Student t test. ADAMTS indicates a disintegrin and metalloproteinase with thrombospondin motifs; AV, aortic valve; LCC, left coronary cusp; NCC, noncoronary cusp; PV, pulmonary valve; and RCC, right coronary cusp. |
|
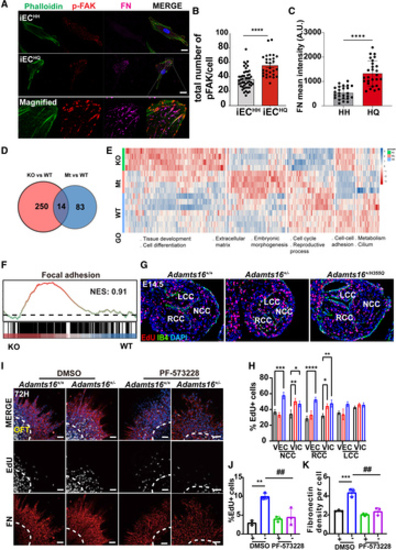
ADAMTS16 deficiency leads to hyperproliferation of valve mesenchymal cells and upregulation of the FAK signaling pathway. A, Immunostaining of phospho-FAK (Tyr397, red), FN (magenta), and phalloidin (green) in iPSC-ECH/H and iPSC-ECH/Q. Scale bars=20 µm. B, Quantification of phospho-FAK (Tyr397) staining in iPSC-ECs. P values were calculated using the Student t test (n=48 for iPSC-ECH/H and n=28 for iPSC-ECH/Q; ****P<0.0001). C, Quantification of FN staining in iPSC-ECs. P values were calculated using the Student t test (n=25 for iPSC-ECH/H and n=27 for iPSC-ECH/Q). ****P<0.0001. D, Venn diagram summarized the 14 common differentially expressed genes (DEGs) among Adamts16+/+, Adamts16+/-, and Adamts16+/H355Q mice. A total of 250 DEGs were identified between Adamts16+/- and Adamts16+/+ mice, and 83 DEGs were discovered between Adamts16+/H355Q and Adamts16+/+ mice. E, Heatmap with cluster and gene set enrichment analysis (GSEA) of DEGs with main biological processes involved in each cluster. F, GSEA of positive regulation of the focal adhesion signaling pathway in Adamts16+/- mice compared with Adamts16+/+ mice. G, Immunostaining of EdU-labeled cells in aortic valves of E14.5 embryos. The EdU-stained cells represented proliferated cells in the valve region. IB4 staining marks valvar endothelial cells (green). Scale bar=100 µm. H, Quantification of EdU-positive cells. The data are presented as the ratio of EdU-positive cells to total valvar endothelial cells or to total valvar interstitial cells in the 3 valve cusps (n=5 for each genotype, and statistical comparison was performed using 2-way ANOVA followed by the Tukey post hoc test). ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05. I, Immunostaining of FN and EdU-labeled cells in outflow tract (OFT) endocardial cushion explants after 72 hours of culture. Either DMSO or a FAK inhibitor (PF-573228, 100 nM) was added to the culture medium for 60 to 72 hours. Scale bars=100 µm. J, Quantification of EdU-positive cells in Adamts16+/+ and Adamts16+/- OFT explants supplemented with or without PF-573228 (n=3). P values were calculated using 2-way ANOVA with the Tukey multiple comparisons test. **P<0.01; ##P<0.01. K, Quantification of fibronectin staining in Adamts16+/+ and Adamts16+/- OFT explants supplemented with or without PF-573228 (n=3). P values were calculated using 2-way ANOVA with the Tukey multiple comparisons test. ##P<0.01; ***P<0.001. ADAMTS indicates a disintegrin and metalloproteinase with thrombospondin motifs; FN, fibronectin; GO, gene ontology; GOBP, GO biological process; KO, Adamts16+/-; LCC, left coronary cusp; Mt, Adamts16+/H355Q; NCC, noncoronary cusp; NES, normalized enrichment score; RCC, right coronary cusp; VECs, valvular endothelial cells; VICs, valvular interstitial cells; and WT, wild-type, Adamts16+/+. |
|
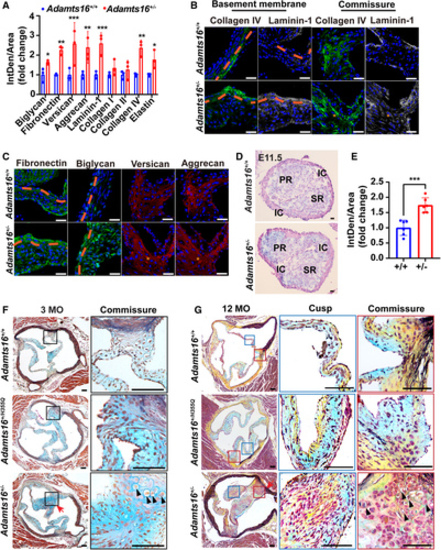
ECM disorganization in Adamts16+/- mouse aortic valves with age. A, Quantification of ECM protein (biglycan, fibronectin, versican, aggrecan, laminin-1, collagen I, collagen II, collagen IV, and elastin) staining in Adamts16+/- and Adamts16+/- mouse aortic valves at P10 (n=3). P values were calculated using the Student t test. ***P<0.001; **P<0.01; *P<0.05. B, Immunostaining of collagen IV and laminin-1 in Adamts16+/+ and Adamts16+/- mouse aortic valves at P10. Orange dashed lines indicate the basement membrane. Scale bars=100 µm. C, Immunostaining of biglycan, fibronectin, versican, and aggrecan in Adamts16+/+ and Adamts16+/- mouse aortic valves at P10. Orange dashed lines indicate the basement membrane. Scale bars=100 µm. D, Alcian blue staining of Adamts16+/- and Adamts16+/- mouse aortic valves at E11.5. Scale bars=100 µm. E, Quantification of Alcian blue staining (n=6). P values were calculated using the Student t test. ***P<0.001. F, Movat’s pentachrome staining of aortic valves in Adamts16+/+, Adamts16+/-, and Adamts16+/H355Q mice at 3 months. Movat’s pentachrome staining identifies elastin fibers as black, collagens as yellow, proteoglycans as blue, muscle as red, and cell nuclei as purple. The red arrow indicates the raphe. Magnified view of the commissure in the black boxed regions. The black arrow in the black boxed region indicates the cartilage-like nodules. Scale bars=100 µm. G, Movat’s pentachrome staining of aortic valves in Adamts16+/+, Adamts16+/-, and Adamts16+/H355Q mice at 12 months. The red arrow indicates commissure fusion (raphe). Magnified view of the valve cusp in the blue boxed regions. Magnified view of the hinge in the red boxed regions. Scale bars=100 µm. ADAMTS indicates a disintegrin and metalloproteinase with thrombospondin motifs; IC, intercalated cushion; MO, month; PR, parietal ridge; and SR, septal ridge. |
|
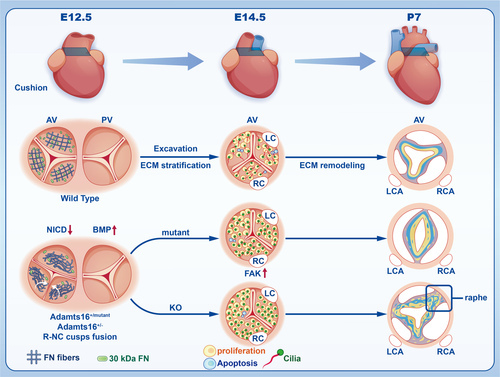
Schematic depicting the deficiency of Adamts16 during valvulogenesis. In the schematic illustration, Adamts16+/+ control mice undergo 3 morphologic stages during valvulogenesis, including endocardial cushion formation (E12.5), cushion elongation (E14.5), and cusp or leaflet remodeling from fetus to adult (postnatal stage). At early E12.5, the cushion separates and develops into 2 tracts, including the aortic valve tract and pulmonary valve tract. At E14.5, the aortic valve exhibits precise regulation of the cell cycle and primary cilia development, providing an optimal developmental environment for valvular cells. After E14.5, the aortic valve continues with elongation and thinning by further valve excavation and ECM remodeling (ie, an organized ECM structure of 3 layers) containing the collagen-rich fibrosa (yellow color), proteoglycan-rich spongiosa (blue color), and elastin-rich ventricularis layers (gray color). However, at E12.5, fibronectin fiber net disassembly and a decreased number of 30-kDa fibronectin fragments occurred in the aortic valve region. At E14.5, abnormal proliferation and apoptosis of VICs and shortening of cilia further disrupted the ECM stratification process and leaflet elongation. At the postnatal stage, the aortic valve presented a disorganized ECM structure in Adamts16-deficient mice, and Adamts16+/- mice exhibited a fused commissure (raphe) accompanied by a loss of ECM trilaminar stratification. ADAMTS indicates a disintegrin and metalloproteinase with thrombospondin motifs; AV, aortic valve; ECM, extracellular matrix; KO, Adamts16+/-; LC, left coronary; LCA, left coronary artery; Mutant, Adamts16+H355Q; PV, pulmonary valve; RC, right coronary; RCA, right coronary artery; and VICs, valvular interstitial cells. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

Unillustrated author statements PHENOTYPE:
|