- Title
-
Repeated sleep deprivation decreases the flux into hexosamine biosynthetic pathway/O-GlcNAc cycling and aggravates Alzheimer's disease neuropathology in adult zebrafish
- Authors
- Park, J., Kim, D.Y., Hwang, G.S., Han, I.O.
- Source
- Full text @ J Neuroinflammation
|
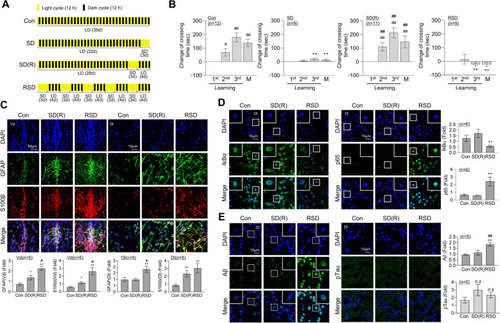
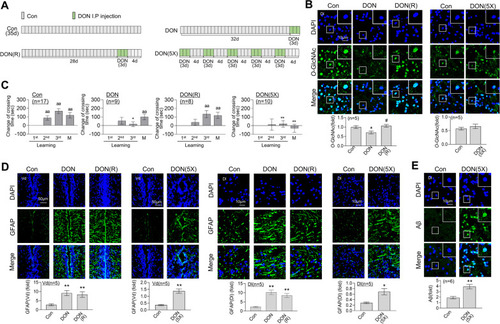
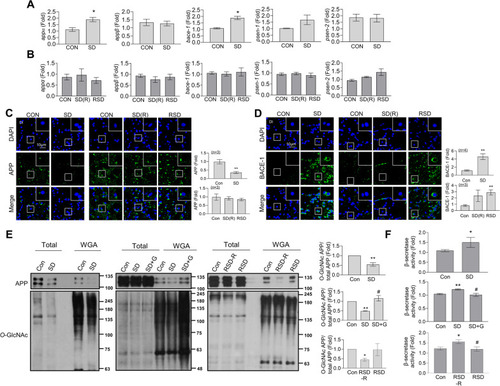
SD- or RSD-induced L/M deficits, neuroinflammation, and Aβ accumulation in the brain of adult zebrafish. For sleep deprivation (SD), zebrafish were exposed to extended light for 72 h (3 days). After SD, the fish had a 4-day recovery period with a normal sleep–wake pattern (SD (R)). Repetitive sleep deprivation (RSD) involved five repeated cycles of SD (R). |
|
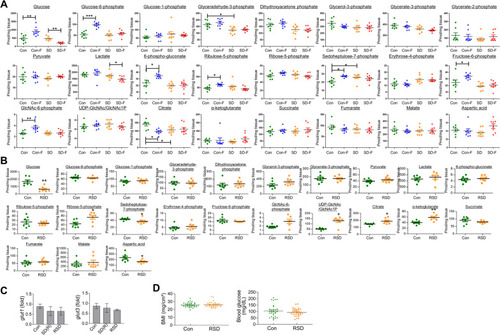
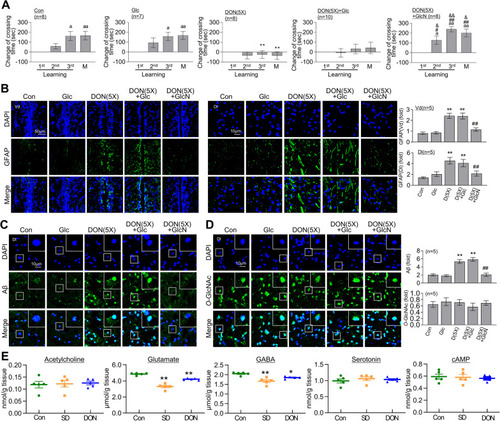
Changes in glucose metabolites in brains of control, SD and RSD zebrafish. |
|
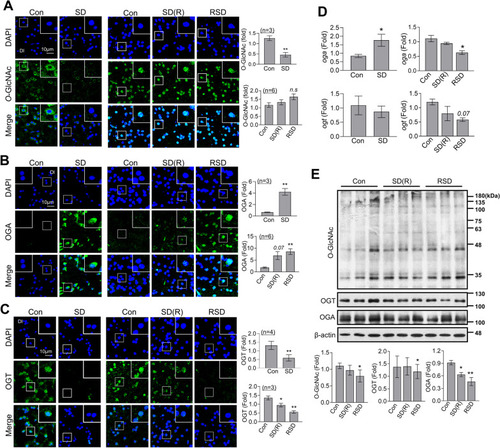
OGT, OGA and |
|
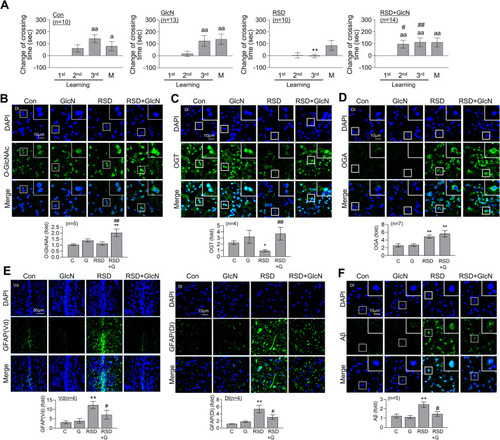
Restoration of RSD-induced L/M deficit, astrocyte activation, and Aβ accumulation by GlcN treatment. Zebrafish were intraperitoneally injected with GlcN (20 μg/g) on the day of SD exposure during each episode of SD throughout 5 cycles of RSD. After the final RSD induction, the fear context L/M test and brain analysis were conducted. |
|
Induction of L/M dysfunction, |
|
Restoration of DON induced L/M defect, astrocyte activation and Aβ accumulation by GlcN. DON (500 ng/g) was intraperitoneally injected for 5 cycles, with each cycle consisting of 3 days of injection followed by 4 days of recovery (DON (5X)). Some groups received an additional intraperitoneal injection of glucose (Glc, 20 μg/g) or GlcN (20 μg/g). After the final cycle of DON injections, fear context L/M test or brain analysis was conducted. |
|
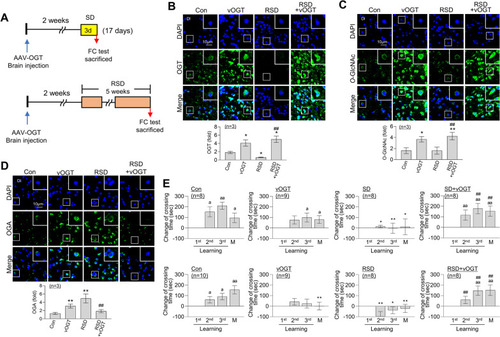
Restoration of RSD-induced L/M deficit by overexpression of OGT. An vOGT virus was injected into the brains of zebrafish. |
|
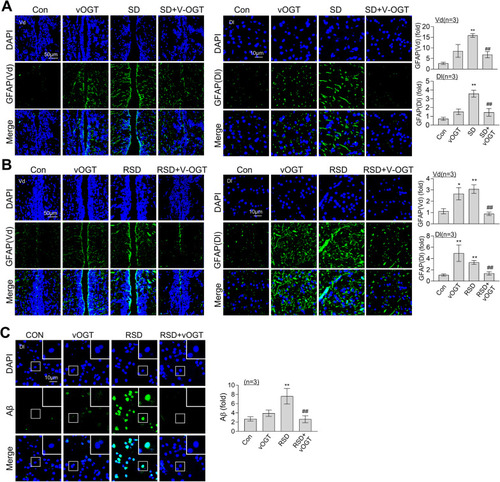
Suppression of RSD-induced astrocyte activation and Aβ accumulation by overexpression of OGT. An vOGT virus was injected into the brains of zebrafish. Representative confocal images (40×) of DAPI (blue), GFAP ( |
|
SD-induced increase in mRNA levels of APP and β-secretase expression, and decrease in APP |