- Title
-
Chlorahololide D, a Lindenane-Type Sesquiterpenoid Dimer from Chloranthus holostegius Suppressing Breast Cancer Progression
- Authors
- Li, Y., Liu, W., Xu, J., Guo, Y.
- Source
- Full text @ Molecules
|
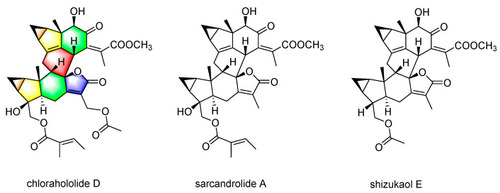
Chemical structure of chlorahololide D, sarcandrolide A, and shizukaol E. |
|
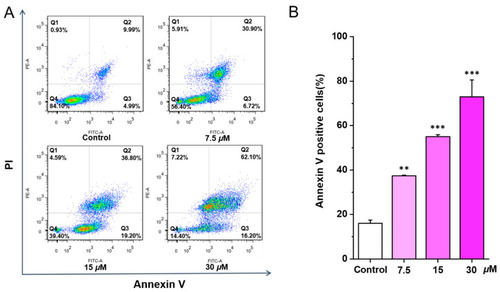
Chlorahololide D triggered apoptosis in MCF-7 cells. Various concentrations of chlorahololide D (7.5, 15, and 30 μM) were administrated to MCF-7 cells and incubated for 48 h. Cells were stained with Annexin V and propidium iodide (PI), and detected by flow cytometry subsequently. ( |
|
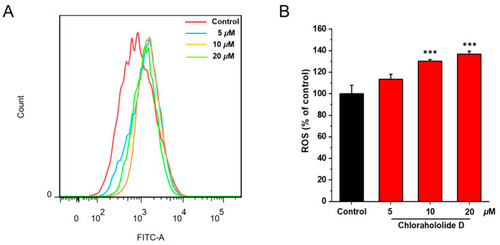
Chlorahololide D increased ROS production in MCF-7 cells. MCF-7 cells were incubated with chlorahololide D (5, 10, and 20 μM) for 48 h. MCF-7 cells were stained with DCFH-DA and detected by the flow cytometer. ( |
|
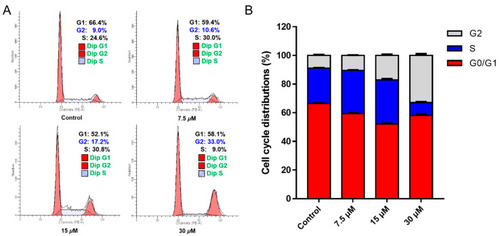
Chlorahololide D arrested G2 phase in MCF-7 cells. MCF-7 cells were treated with chlorahololide D (7.5, 15, and 30 μM) for 48 h. ( |
|
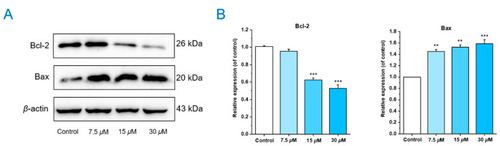
Chlorahololide D regulated apoptosis-related proteins. MCF-7 cells were pre-treated with chlorahololide D for 36 h, and Western blotting analysis was carried out. ( |
|
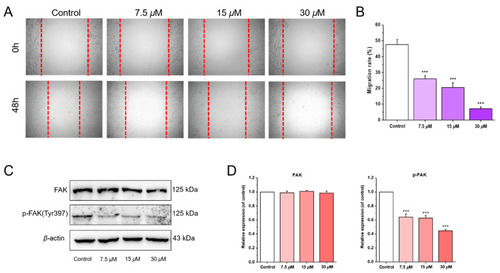
Chlorahololide D inhibited MCF-7 cells migration via regulating migration-related proteins. MCF-7 cells were pre-treated with chlorahololide D for 48 h, and Western blotting analysis was carried out. ( |
|
Chlorahololide D inhibited proliferation and migration |
|
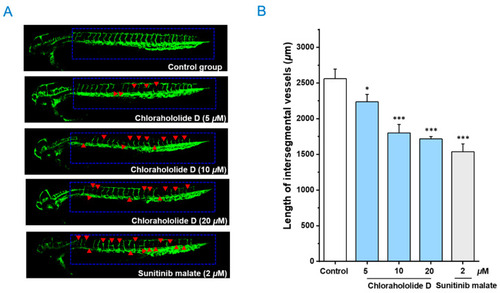
The embryos of transgenic zebrafish |