- Title
-
A role for dopamine in control of the hypoxic ventilatory response via D2 receptors in the zebrafish gill
- Authors
- Reed, M., Pan, W., Musa, L., Arlotta, S., Mennigen, J.A., Jonz, M.G.
- Source
- Full text @ J. Comp. Neurol.
|
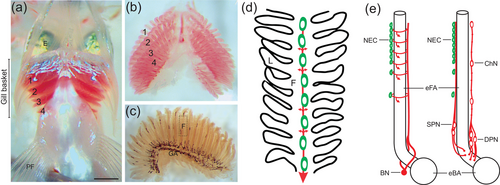
Illustration of the whole-mount gill preparation in zebrafish and organization of the structures involved in oxygen sensing. (a) Gill baskets were removed ventrally from zebrafish, as shown. Rostral is at the top of the image and the gill filaments of gill arches 1−4 are labeled. E, eye; PF, pectoral fin. The scale bar represents 0.5 mm and applies to panels (b) and (c). An isolated gill basket is shown in panel (b), and a single gill shown in panel (c). GA, gill arch. The dashed box indicates a typical region of a gill filament (F) that was studied using confocal microscopy and is represented as a schematic in panel (d). (d) In this simplified representation, oxygen-sensitive neuroepithelial cells (NECs, green) are located along the filament (F) and receive sensory innervation (red). Nerve fibers form a plexus (not shown) around NECs and extend laterally to the secondary lamellae (L, small arrows), and large nerve bundles (large arrow) extend toward the gill arch. (e) Extrabrancial (left) and intrabranchial (right) innervation of filament NECs arise from the branchial nerve (BN) or superficial and deep proximal neurons (SPN and DPN), respectively. E is modified from Jonz and Nurse (2008), with permission from the authors. |
|
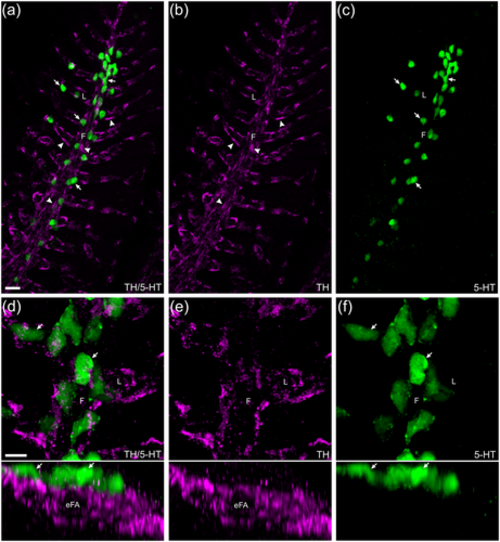
Confocal imaging of immunohistochemical localization of tyrosine hydroxylase (TH)-positive nerve fibers associated with serotonergic neuroepithelial cells (NECs). (a) NECs (arrows) labeled with anti-serotonin (5-HT, green) were closely associated with a plexus of nerve fibers (arrowheads) labeled by anti-TH (magenta) in the filaments (F) and lamellae (L). (b, c) TH and 5-HT labeling shown separately. (d–f) Images of gill filaments and lamellae are shown at higher magnification (upper panels) and tilted back 90° (lower panels). In the latter, the transverse optical section revealed the close association between NECs and nerve fibers, and that the nerve fibers surrounded the efferent filament artery (eFA). Scale bar in panel (a) = 20 μm and applies to panels (b) and (c). Scale bar in panel (d) = 20 μm and applies to panels (e) and (f). |
|
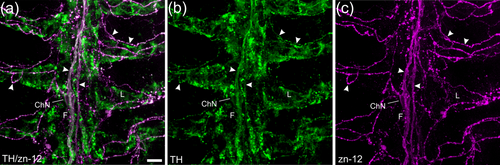
Confocal imaging of immunohistochemical co-localization of tyrosine hydroxylase (TH) with a zebrafish-specific neuronal marker. (a) Labeling with anti-TH (green) co-localized with nerve fibers labeled with zn-12 (magenta). (b, c) TH and zn-12 labeling shown separately. Both nerve bundles of the central filament (F) and all zn-12-positive nerve fibers (arrowheads) of the filaments and lamellae (L) were also TH positive. Some labeling by anti-TH did not co-localize with zn-12. An intrabranchial chain neuron (ChN) is indicated that was labeled with both markers. Scale bar in panel (a) = 10 μm and applies to all panels. |
|
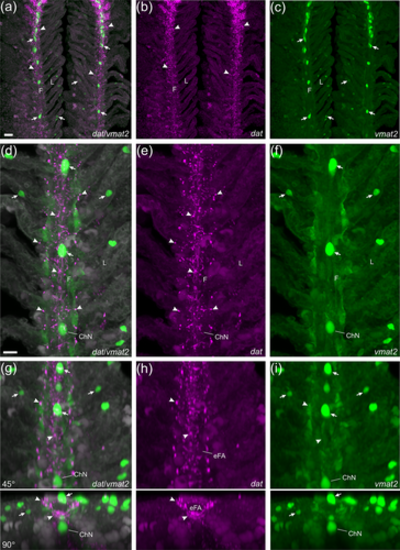
Confocal imaging of nerve fibers expressing the dopamine active transporter (dat) and associated neuroepithelial cells (NECs) expressing the vesicular monoamine transporter (vmat2) in gills of the Tg(dat:mCherry)/ET(vmat2:GFP) line. (a) dat |
|
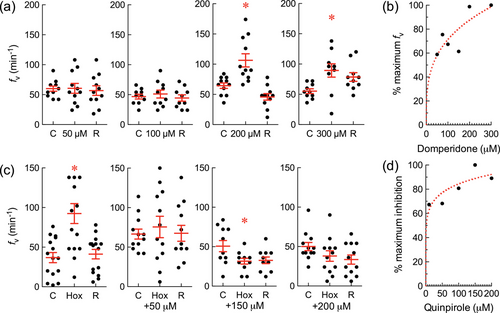
In chemical screening assays, domperidone (a dopamine D2 receptor antagonist) increased breathing frequency (fv), and quinpirole (a dopamine D2 receptor agonist) abolished the hyperventilatory response to hypoxia. fv |
|
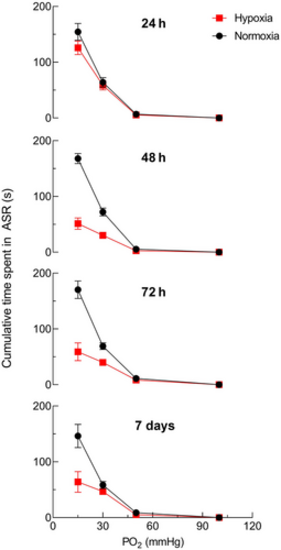
Acclimation to chronic hypoxia reduced the aquatic surface respiration (ASR) response to acute hypoxia. Adult zebrafish previously acclimated to hypoxia for various timepoints (24 h, 48 h, 72 h, or 7 days) were exposed to successive bouts of progressively more severe acute hypoxia (100, 50, 30, and 15 mmHg). Data points for all groups were connected with a continuous line for clarity. Acclimation to hypoxia for 48 h, 72 h, and 7 days significantly reduced mean ± SEM cumulative time in ASR during acute hypoxic exposure (squares) at Po2 values below 30 mmHg, compared with unacclimated zebrafish (circles; Mann–Whitney U test; p < .01; N = 8 in control and acclimated groups for each Po2 and each period of acclimation). |
|
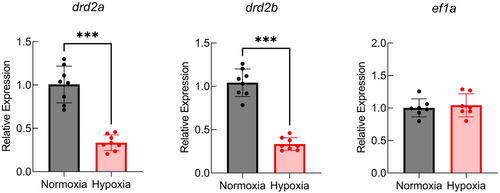
Relative gene expression of drd2a and drd2b (encoding D2 receptors) decreased following 48 h of chronic hypoxia. Data were normalized to the mRNA abundance of the reference gene, ef1a. Data were analyzed using a Mann–Whitney U |
|
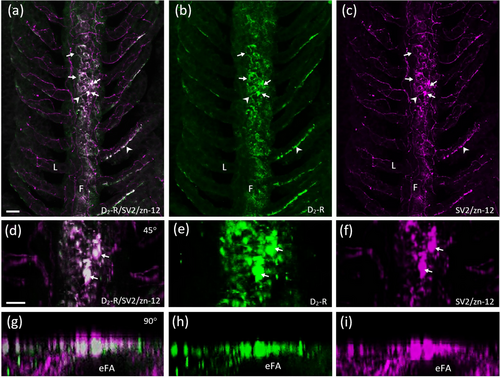
Characterization of D2-positive cells of the efferent gill epithelium. Confocal imaging of immunohistochemical labeling of dopamine receptor 2 (D2-R) with a zebrafish-specific neuronal marker (zn-12) and neuroepithelial cells (NECs) containing synaptic vesicle protein-2 (SV2). (a) Labeling with anti-D2-R (green) co-localized with NECs (arrows) labeled with SV2 (magenta) and nerve fibers labeled with zn-12 (magenta) in the filaments (F). (b, c) D2-R and SV2/zn-12 labeling shown separately. Some zn-12-positive nerve fibers (arrowheads) of the filament and lamellae (L) were also D2-R positive. (d–f) Co-localization of NECs and D2-R from panels (a–c) shown at higher magnification and tilted back 45°. (g–i) Images from panels (a–c) tilted back 90°. Rotation demonstrates D2-R-positive NECs in a transverse optical section oriented superficial to the efferent filament artery (eFA). Scale bar in panel (a) = 20 μm and applies to panels (b) and (c). Scale bar in panel (d) = 20 μm and applies to panels (e–i). |
|
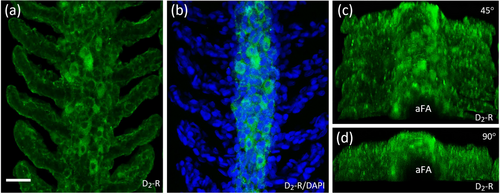
Characterization of D2-positive cells of the afferent gill epithelium. (a) Confocal imaging of immunohistochemical localization of dopamine receptor 2 (D2-R) on the afferent filament epithelium. (b) Co-application with DAPI-labeled nuclei of D2 |
|
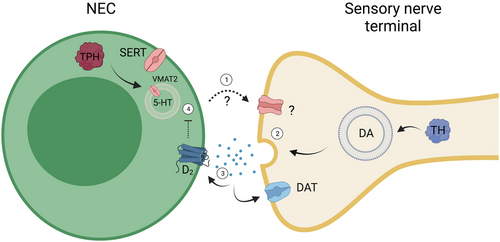
Model of the proposed role for dopamine in modulating the hypoxic response in gill neuroepithelial cells (NECs) of the efferent epithelium. As shown in the present study, postsynaptic nerve terminals in the gill filaments express tyrosine hydroxylase (TH) and the dopamine active transporter (DAT), involved in synthesis and uptake of dopamine (DA), respectively. When the NEC is stimulated during hypoxia: (1) Release of an unknown neurotransmitter from the NEC activates a postsynaptic receptor on the sensory nerve terminal. (2) Dopamine is then released from the nerve terminal and (3) activates presynaptic D2 receptors of the NEC, or is taken up postsynaptically by DAT. (4) The former leads to inhibition of the NEC response to hypoxia. The excitatory neurotransmitter released by NECs has not been confirmed but may be serotonin (5-HT). As illustrated, zebrafish NECs contain 5-HT, synaptic vesicles, and the vesicular monoamine transporter, VMAT2 (Jonz & Nurse, 2003; Pan et al., 2021). NECs also express genes encoding tryptophan hydroxylase and the serotonin transporter (SERT) for possible 5-HT synthesis and uptake (Pan et al., 2022). Created with BioRender.com. |