- Title
-
Noradrenergic tone is not required for neuronal activity-induced rebound sleep in zebrafish
- Authors
- Benoit, E., Lyons, D.G., Rihel, J.
- Source
- Full text @ J. Comp. Physiol. B
|
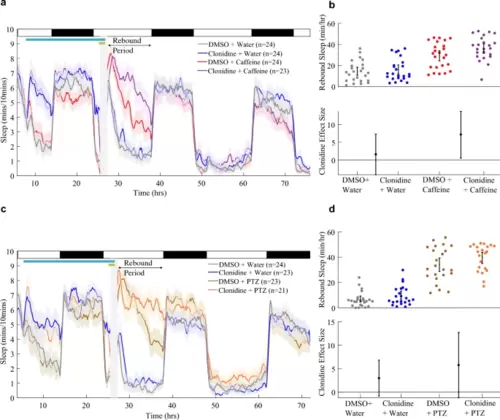
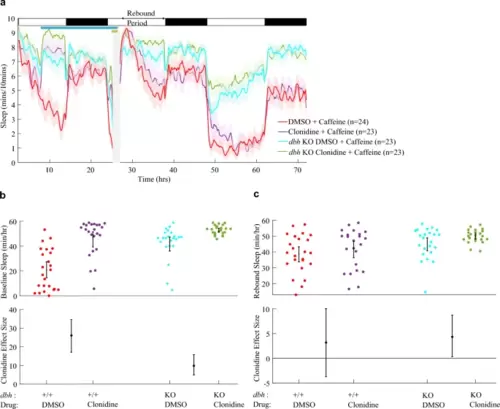
Activating α2-adrenoceptors during drug-induced arousal facilitates rebound sleep. a Sleep traces (± SEM) beginning at 5 dpf and continuing over three days and nights (time since ZT0 at 5 dpf) for larvae exposed to combinations of 5 µM clonidine/DMSO and 2 mM caffeine/water. Following drug wash-off, larvae experience rebound sleep (labelled Rebound Period). At the top, white and black bars represent day and night, respectively; the pale blue horizontal bar shows the clonidine exposure window, while the gold bar indicates the presence of stimulant. b Upper chart shows the average total sleep/h during the rebound period for each larva (black bar: mean and 95% CI). Lower chart shows the effect size (with 95% CI) of clonidine treatment on boosting rebound sleep/h among water-treated and caffeine-treated groups. c Sleep traces as in a for larvae exposed to combinations of clonidine and 10 mM PTZ. The post-drug rebound sleep period of c is summarised for each larva in d (upper chart). Lower chart shows the effect size (with 95% CI) of clonidine treatment on boosting rebound sleep/h among water-treated and PTZ-treated groups |
|
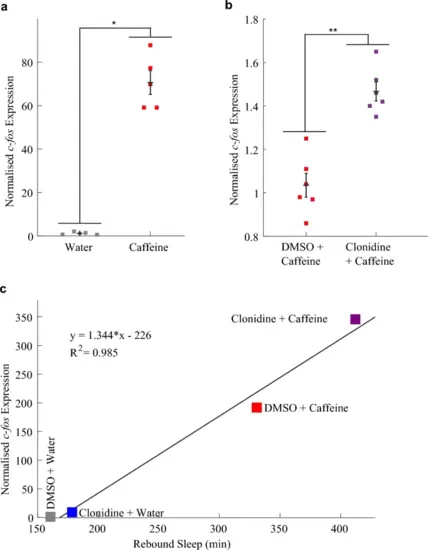
c-fos expression is higher in larvae following combined treatment with clonidine and caffeine than following caffeine alone. a qRT-PCR on groups of ~ 20 larvae (n = 4 and n = 5 biological replicates per condition) reveals that larvae treated with caffeine had a significant, 71-fold increase in c-fos expression compared to water-treated larvae (*p < 0.05, two tailed Wilcoxon rank sum test performed on the “dCt” metric, see Fig. S3a). b c-fos expression of larvae soaked in clonidine before and during caffeine exposure was significantly higher by 47% than in larvae exposed to caffeine alone (n = 6 biological replicates per condition, **p < 0.01, see Fig. S3b). c The relative c-fos expression induced by different combinations of vehicle, clonidine and caffeine is positively, linearly correlated (R2 = 0.985) with the total rebound sleep induced by these drugs. qRT-PCR was performed on groups of 37 larvae (see Fig. S3c). Note that c plots together the results of two separate experiments; in both experiments there were four groups of larvae each treated with one of the four combinations of clonidine, caffeine, DMSO and water, but in one experiment c-fos expression was measured after drug treatment, and in the other rebound sleep was measured (sleep data is per Fig. 1a and b). Each square in a–c is the mean of three technical replicates |
|
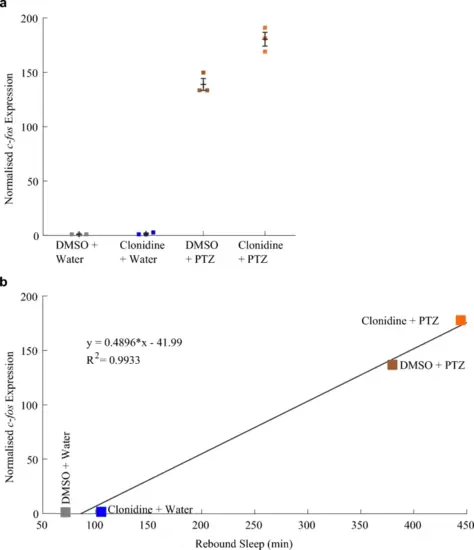
Rebound sleep levels correlate with c-fos expression across different clonidine/PTZ treatment combinations. a qRT-PCR on groups of ~ 17 larvae (n = 3 biological replicates per condition) reveals that larvae treated with both clonidine and PTZ had a trend towards higher c-fos expression than those treated with PTZ alone (see also Fig. S4a). b The mean c-fos expression induced by each drug combination is strongly positively correlated (R2 = 0.993) with the amount of rebound sleep induced by each drug condition (see Fig. 1c and d). Each square in a is the mean of three technical replicates |
|
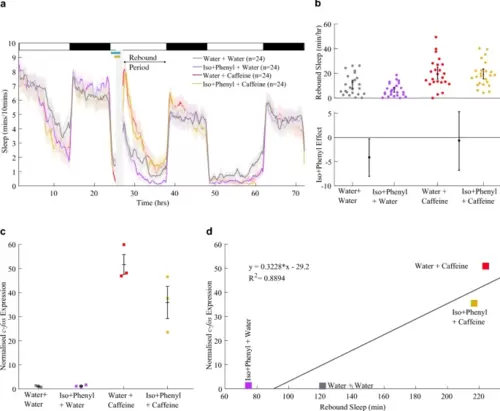
Activating noradrenergic transmission with isoproterenol and phenylephrine marginally depresses caffeine-induced c-fos expression. a Sleep traces for larvae exposed to combinations of 10 µM isoproterenol + 10 µM phenylephrine (“Iso + Phenyl”) and/or caffeine. At top left, the pale blue horizontal bar shows the isoproterenol + phenylephrine exposure window while the gold bar indicates the presence of caffeine. The post-drug rebound sleep period of a is summarised for each larva in b (upper chart). Lower chart shows the effect size (with 95% CI) of Iso + Phenyl treatment among water-treated and caffeine-treated groups. c qRT-PCR on groups of ~ 18 larvae reveals that each group of larvae pre-treated with isoproterenol + phenylephrine and then caffeine (n = 3 biological replicates) had lower relative c-fos expression than the groups of larvae treated with water and then caffeine (n = 3 biological replicates); see also Fig. S5a. Each square is the mean of three technical replicates. d The average relative c-fos expression induced by each condition is strongly positively correlated (R2 = 0.889) with the total rebound sleep that was induced by the same drug condition (from a and b) |
|
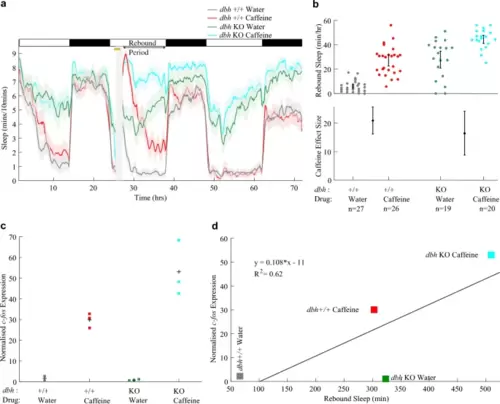
Caffeine triggers c-fos expression more strongly in dbh F0 KOs. a Sleep traces for dbh F0 KO and control-injected larvae exposed to either caffeine or water. The post-drug rebound sleep period of a is summarised for each larva in b (upper chart). Caffeine treatment had a strong boosting effect on rebound sleep in both control-injected and dbh F0 KO larvae (lower chart). The caffeine effect size is not significantly different between the two genotypes. c qRT-PCR on groups of ~ 15 larvae revealed that each group of dbh F0 KO larvae treated with caffeine (n = 3 biological replicates) showed greater relative c-fos expression than the groups of control larvae treated with caffeine (n = 3 biological replicates); see also Fig. S9a. Each square is the mean of triplicate technical replicates. d There is a weak positive correlation between c-fos expression and subsequent rebound sleep levels (R2 = 0.62), but water-treated dbh F0 KO larvae do not conform to this correlation, showing high sleep levels despite low c-fos expression |
|
Clonidine enhances sleep, and rebound sleep, in dbh F0 KO larvae. a Sleep traces for dbh F0 KO and control-injected larvae exposed to clonidine/DMSO and caffeine. b At 5 dpf from clonidine treatment until lights-out, clonidine had a positive effect on sleep levels in both control-injected and dbh F0 KO larvae, as illustrated by positive effect sizes and 95% CIs. The effect size of clonidine was greater among control-injected larvae. The post-caffeine rebound sleep period is summarised for each larva in c. Among dbh F0 KOs, clonidine had a positive effect on rebound sleep. The clonidine effect size was not significantly different between the two genotypes. Deep sequencing was used to verify the successful loss-of-function targeting of dbh in 10 randomly selected dbh F0 KO larvae: all animals had > 93% (mean, 96%) of their amplified dbh copies frameshifted (see Fig. S10) |
|
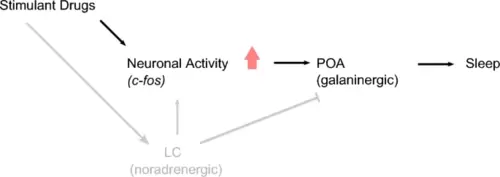
Noradrenergic activity is not required for stimulant-induced c-fos expression and rebound sleep. During waking, the LC releases noradrenalin to brain-wide targets, promoting arousal (Carter et al. 2010) and inhibiting sleep-promoting GABAergic/galaninergic neurons of the POA (Liang et al. 2021). Despite the role of the LC in maintaining arousal and heightened neuronal activity during waking, our results suggest that stimulant-induced neuronal activity and rebound sleep can occur in the absence of prior noradrenergic tone. Building on the work of Reichert et al. (2019), we propose a model in which stimulant-induced increases in neuronal activity subsequently promote activation of GABAergic/galaninergic sleep-promoting neurons of the POA, which drive sleep, independently of noradrenergic activity. Arrowheads denote activating projections; the bar head denotes an inhibitory projection |