- Title
-
A multilevel screening pipeline in zebrafish identifies therapeutic drugs for GAN
- Authors
- Lescouzères, L., Hassen-Khodja, C., Baudot, A., Bordignon, B., Bomont, P.
- Source
- Full text @ EMBO Mol. Med.
|
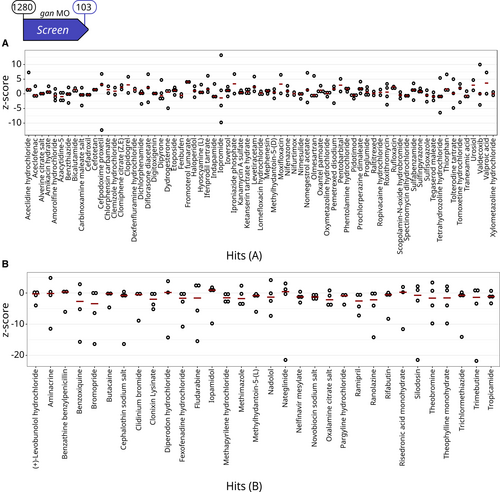
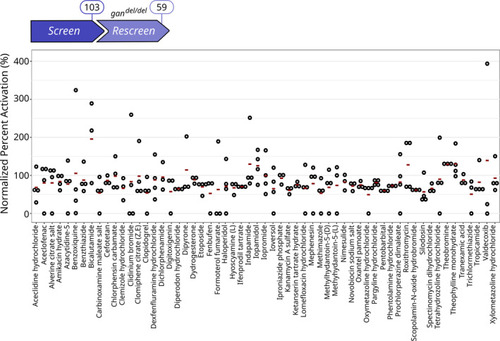
Primary Screen identifies 103 compounds restoring locomotion defects in the gan MO-injected zebrafish A, B. Single z-scores, comparing the total distance traveled by quadruplicate gan fish over 1-h period are plotted for the 71 A Hits (A) and the 32 B Hits (B). Data information: For each compound, circles represent individual larvae (n = 4 per drug) and red lines show the median z-scores (see Fig EV2 for results on the whole library). |
|
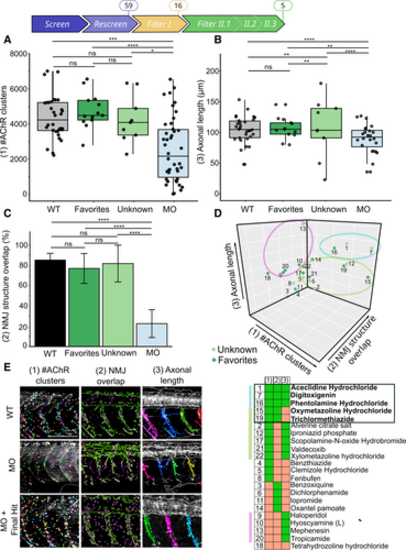
Filter II: The automated imaging-analysis identifies five Hits regulating NMJ development in the gan zebrafish A–C. Boxplots showing individual and mean values of the number of postsynaptic AChR clusters (A) and axonal length (B) and bar plot showing NMJ structure overlap (C) for four groups: noninjected WT (black), MO-injected embryos (blue), MO-injected embryos treated with Favorites Hits (dark green) or Unknown Hits (light green). The central bands of the boxplots represent the median, the boxes of the boxplots represent the interquartile range (between the first and third quartile), and the whiskers represent the minimum and maximum values. D. 3-D scatter plot representing the z-scoring analysis for the three parameters (x (1) = #AChR clusters, y (2) = NMJ structure overlap, z (3) = Axonal length). The 3-D representation and the associated Hit-map identify subgroups of compounds with different penetrance of recovery of cellular deficits in the gan zebrafish, among which three Hits restore all parameters and two additional restore two parameters including NMJ common to all Hits. E. Representative images of the three parameters examined for noninjected WT embryos (WT), gan MO-injected embryos (MO), and gan MO-injected embryos treated with Aceclidine Hydrochloride, which restore all three parameters. Data information: Scale bar represents a length of 200 μm. Data information: Each dot represents individual values for WT and MO, and mean values of quadruplicate treated larvae with single Hits (Favorites, Unknown) (A–C). In the absence of normality of distribution of the data, a nonparametric Kruskal–Wallis test is applied; medians with range are represented; n = 34 (WT), n = 44 (MO), n = 13 (Favorites), n = 9 (Unknown); *P ≤ 0.05; **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001. |
|
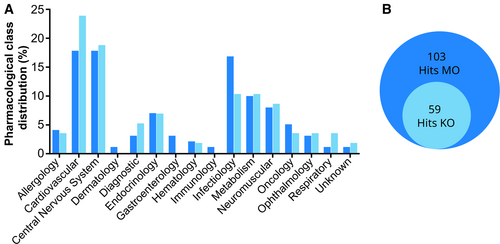
Pharmacological class of the Hits A, B. Bar plot (A) showing the distribution of the therapeutic classes (%) of the 59 Hits common to the gan morphants and the gan KO line (B), according to the Prestwick Chemical Library annotations. |
|
Additional AChR-related phenotypes in the gan zebrafish, which are not rescued by the five Final Hits A–D. Boxplots showing individual and mean values of the labeling intensity of presynaptic (stained with Synaptic Vesicle glycoprotein 2 (SV2)) (A) and postsynaptic (stained with α-bungarotoxin (αBTX)) (B) AChR clusters and area of post-synaptic AChR (D) for three groups: noninjected WT (black), MO-injected embryos (blue), MO-injected embryos treated with the five Final Hits (dark green), as identified in Fig 7. (C) Representative images of SV2 intensity labeling for noninjected WT embryos (WT), gan MO-injected embryos (MO) and gan MO-injected embryos treated with Phentolamine Hydrochloride. Analysis performed at 48 hpf. Scale bar represents a length of 500 μm. Each dot represents individual values for WT (n = 6 (A, B), n = 34 (D)) and MO (n = 6 (A, B), n = 43 (D)), and mean values of quadruplicate treated larvae (n = 5 (A, B, D)) with single Hits (A, B, D). The central bands of the boxplots represent the median, the boxes of the boxplots represent the interquartile range (between the first and third quartile), and the whiskers represent the minimum and maximum values. In the absence of normality of distribution of the data, a nonparametric Kruskal–Wallis test is applied; medians with range are represented; **P ≤ 0.01, ***P ≤ 0.001. |
|
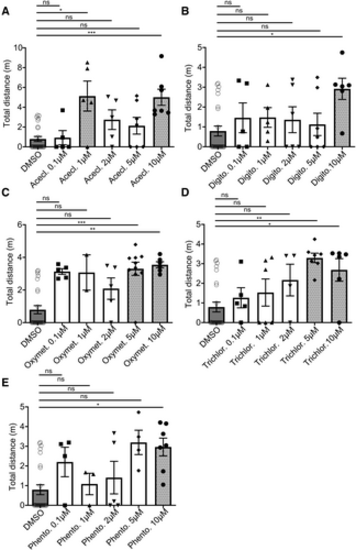
Dose–response effect of the five favorite Hits on the restoration of motility A–E. Treatment with Aceclidine Hydrochloride (Acecl.) (A), Digitoxigenin (Digito.) (B), Oxymetazoline Hydrochloride (Oxymet.) (C), Trichlormethiazide (Trichlor.) (D) and Phentolamine Hydrochloride (Phento.) (E) was performed from 0.1 to 50 μM. Data information: Data are not provided for the higher doses (30 and 50 μM) due to toxicity. Results show the distance traveled at 5 dpf, during 1 h by gan MO-injected larvae, treated with either DMSO or Hits from 0.1 to 10 μM. Each dot represents individual values, (A) n = 25 (MO + DMSO), n = 5 (MO + Acecl. 0.1 μM), n = 5 (MO + Acecl. 1 μM), n = 5 (MO + Acecl. 2 μM), n = 7 (MO + Acecl. 5 μM), n = 7 (MO + Acecl. 10 μM); (B) n = 25 (MO + DMSO), n = 5 (MO + Digito. 0.1 μM), n = 5 (MO + Digito. 1 μM), n = 6 (MO + Digito. 2 μM), n = 7 (MO + Digito. 5 μM), n = 5 (MO + Digito. 10 μM); (C) n = 25 (MO + DMSO), n = 5 (MO + Oxymet. 0.1 μM), n = 2 (MO + Oxymet. 1 μM), n = 5 (MO + Oxymet. 2 μM), n = 10 (MO + Oxymet. 5 μM), n = 6 (MO + Oxymet. 10 μM); (D) n = 25 (MO + DMSO), n = 5 (MO + Trichlor. 0.1 μM), n = 6 (MO + Trichlor. 1 μM), n = 4 (MO + Trichlor. 2 μM), n = 6 (MO + Trichlor. 5 μM), n = 6 (MO + Trichlor. 10 μM); (E) n = 25 (MO + DMSO), n = 4 (MO + Phento. 0.1 μM), n = 3 (MO + Phento. 1 μM), n = 5 (MO + Phento. 2 μM), n = 4 (MO + Phento. 5 μM), n = 7 (MO + Phento. 10 μM). In the absence of normality of distribution of the data, a nonparametric Kruskal–Wallis test is applied; means ± SEM are represented. *P ≤ 0.05; **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001. |
|
Beneficial effect of the Hits upon treatment at symptomatic stage A. Phenotype of gan MO-injected embryos at 48 hpf, with deficits in touch-responsiveness and impairment of axons and neuromuscular junctions (znp1: green; αBTX: α-bungarotoxin: red). Scale bar represents a length of 100 μm. B–E. Restoration of motility of 5-day-old gan larvae, when treated from 48 hpf with Aceclidine Hydrochloride (Acecl.) (B), Oxymetazoline Hydrochloride (Oxymet.) (C), Trichlormethiazide (Trichlor.) (D) and Phentolamine Hydrochloride (Phento.) (E). Hits were applied from 5 to 30 μM concentrations, with daily bath changes; data are not provided for the higher doses (20 and 30 μM) due to toxicity. Results show the distance traveled during 1 h by gan MO-injected larvae, treated with either DMSO or Hits at 5 and 10 μM. In the absence of normality of distribution of the data, a nonparametric Kruskal–Wallis test is applied; means ± SEM are represented. **P ≤ 0.01. |
|
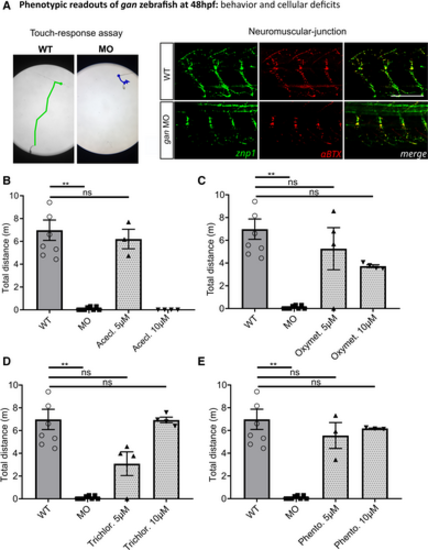
The Schematic of the behavioral and cellular defects described in the The percentage of shorter pMN axons (< 70 μm) per fish is significantly higher in Representative images for the neuromuscular junctions (znp1: green; αBTX: α‐bungarotoxin: red) in WT and Representation of the cumulative tracking of the spontaneous locomotion of 5‐day‐old larvae for 1 h, in noninjected and MO‐injected animals. Quantitative measures of the traveled distance (m: meter) show total loss of locomotion in 79.2% of Data information: (B, E) Each dot represents individual larvae; * |
|
Development of a screening strategy in zebrafish to score, categorize, and refine candidates to 103 hits Efficacy of Hits is determined by the establishment of a z‐score scale, ranging from 0 (noninjected “WT Control,” green) to negative values < −2 ( Primary screening on Summary of the screening strategy workflow for the GAN disease, encompassing behavioral tests in the two |
|
Rescreen identifies 59 compounds restoring locomotion defects in the The 103 Hits identified in the |
|
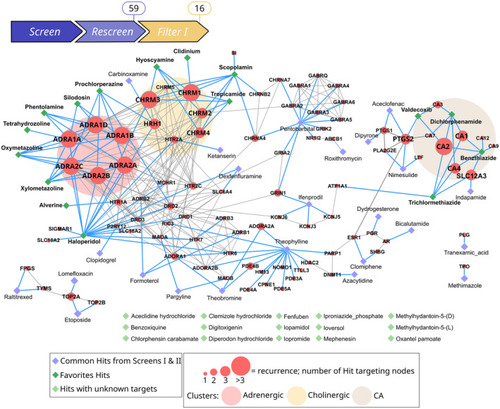
Filter I: Computational network analysis of the Hit targets identifies three functional groups and high recurrence, and refines candidates to 16 Hits Drug–Target list was constructed with known Drug–Target Interaction data extracted from DrugBank for the 59 candidate Hits. Subsequently, gene network of the 89 targets and interactions of the corresponding proteins were obtained from the STRING database (non‐interacting protein are removed here). Diamond‐shaped nodes correspond to drugs compounds as annotated in the DrugBank database and red nodes correspond to their targets (gene names presented here). Isolated diamonds (in light green) correspond to the 15 drugs with unknown targets. Edges corresponding to Target–Target interactions from STRING are indicated as solid gray lines, and Drug–Target interactions from DrugBank in blue lines. Recurrence analysis pinpoints a clustering of Hits into three functional groups with high recurrence, as depicted with ellipses: Adrenergic (light pink), Cholinergic (light yellow), CA (light brown). Target node size is scaled according to the number of drugs targeting the node and reflects recurrence. Recurrence of > 3 refines favorite drugs to 16 Hits (dark green diamond), represented in the three functional groups. |
|
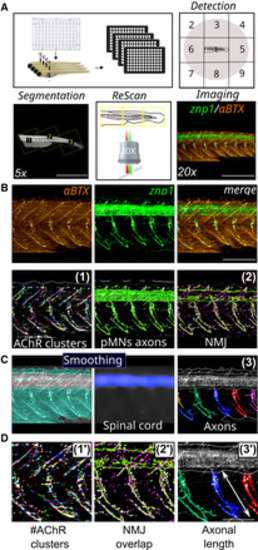
Filter II: Novel methodological analysis of automated imaging‐screening Schematic overview of 48‐h‐old embryos for automated detection in 96 well‐plates, ROI segmentation and Rescan imaging. Representative images of NMJ staining (znp1: green; αBTX: α‐bungarotoxin: red) within the spinal cord of control larvae. Representative images of the detection filters segmenting AChR clusters from α‐bungarotoxin staining (1), pMNs axon area from znp1 staining and NMJ overlapping compounds (2) within the spinal cord of control larvae. Representative image of the smoothing filter to extract the dorsal spinal cord staining as the specific region of interest to individualize axons (3). Enlarged pictures of the parameters (1′–3′) for the quantification of NMJ and pMN axons. Data information: Scale bar represents a length of 1 mm (A, 5×), 500 μm (A, 20×), 200 μm (B), 100 μm (D). |
|
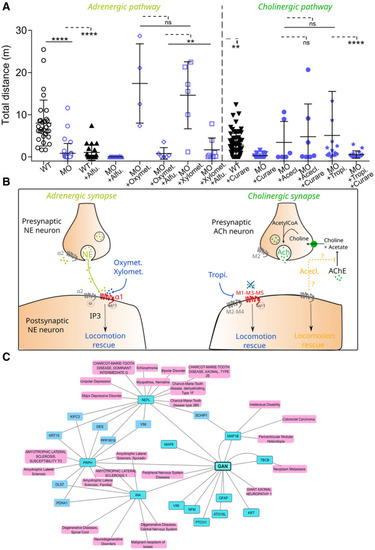
Specificity of the Hits for on‐target effects at the NMJid='emmm202216267-li-0028'> Box plots representing the effect of adrenergic (left, Oxymet., Oxymetazoline Hydrochloride; Xylomet., Xylometazoline Hydrochloride) and cholinergic (right, Acecl., Aceclidine Hydrochloride; Tropi., Tropicamide) Hits, in the presence of pathway‐specific blockers (Alfu., Alfuzosin, Curare) added as pretreatment. Each dot represents individual values for non‐injected WT embryos, MO‐injected larvae and MO‐injected larvae treated with the Hits ± blockers. In the absence of normality of distribution of the data, a nonparametric Kruskal–Wallis with Dunn's multiple comparison test is applied; means with ± standard deviation are represented; Proposed mechanisms of action of the adrenergic (left) and cholinergic (right) Hits on associated synapses. G‐coupled receptors are represented at the pre‐ or postsynaptic membrane according to their localization. Hits whose locomotion rescue action is expected to be dependent on direct action on receptors are shown in blue. Conversely, Hit whose action is assumed to be independent of its role as an agonist is shown in orange. NE, Norepinephrin; ACh, Acetylcholine; AChE, Acetylcholine‐esterase. Interaction's network between GAN and human diseases. Gigaxonin's substrates (dark blue) and associated diseases (light pink) were manually added to the initial list. The gene‐disease‐associated interactions with (KRT15, VIM, DES) are not displayed because too numerous, but they seem principally associated with cancer. The network of diseases closest to GAN mainly includes NMD. We distinguish several groups of neuromuscular pathologies, including numerous CMT forms, motor neuron pathology (ALS) and myopathies. |
|
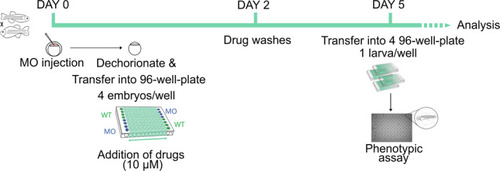
Schematic representation of the timeline and workflow of the drug screening in the At 0 hpf, WT eggs are injected with |
|
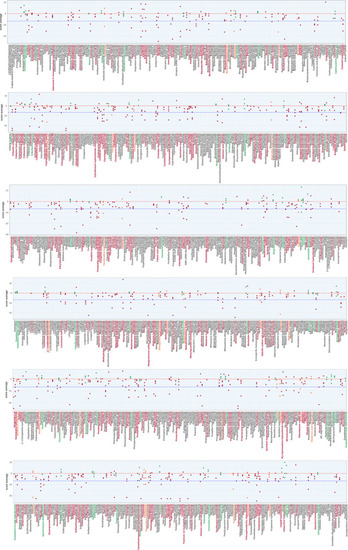
Exhaustive representation of the z‐scores obtained in the Screen for the 1,280 compounds For each drug, individual z‐scores of quadruplicate fish are plotted and assessed for the total distance traveled at 5 dpf by |