- Title
-
Modeling Lung Carcinoids with Zebrafish Tumor Xenograft
- Authors
- Carra, S., Gaudenzi, G., Dicitore, A., Cantone, M.C., Plebani, A., Saronni, D., Zappavigna, S., Caraglia, M., Candeo, A., Bassi, A., Persani, L., Vitale, G.
- Source
- Full text @ Int. J. Mol. Sci.
|
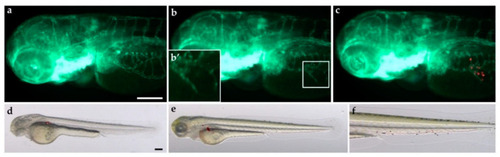
Engraftment of lung carcinoid cells in zebrafish embryos. Representative epifluorescence images of |
|
Lung carcinoid cell invasiveness in grafted zebrafish embryos. Overlay of representative fluorescent and bright field images of embryos grafted with red-stained NCI-H835, UMC-11, NCI-H727, and NCI-H720 cells at 0 ( |
|
Tumorigenic potential of a lung carcinoid PDX in zebrafish embryos. Red-stained cells, obtained from a patient surgical resection, were used to perform PDX in 48 hpf |
|
Time-lapse imaging of lung carcinoid cell grafted embryos performed with selective plane illumination microscopy. Representative maximum intensity projections of volumetric stacks acquired of |
|
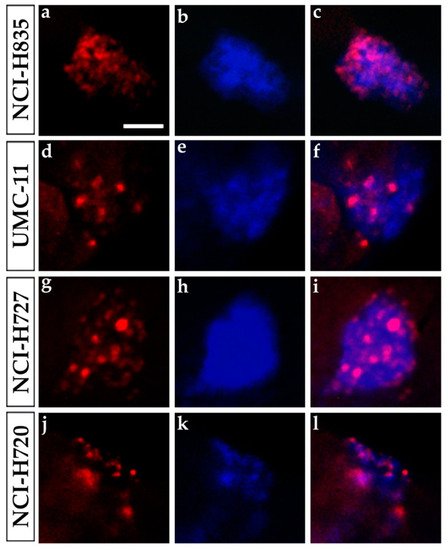
Ki-67 immunostaining of lung carcinoid grafted embryos. Representative images of 48 hpi |
|
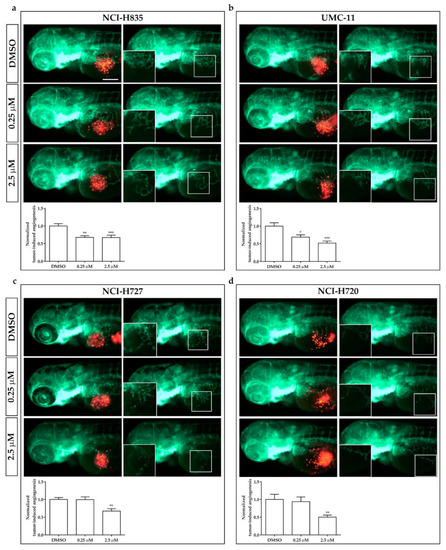
Effects of sulfatinib on tumor-induced angiogenesis in zebrafish embryos implanted with lung carcinoid cells. Representative epifluorescence images of |
|
Effects of sulfatinib treatments on the invasiveness of lung carcinoid cells in grafted zebrafish embryos. Quantification of cell spread in the tail of embryos injected with NCI-H835 ( |