- Title
-
Promoter Binding and Nuclear Retention Features of Zebrafish IRF Family Members in IFN Response
- Authors
- An, L.L., Zhao, X., Gong, X.Y., Li, Y.L., Qu, Z.L., Sun, H.Y., Guo, W.H., Dan, C., Gui, J.F., Zhang, Y.B.
- Source
- Full text @ Front Immunol
|
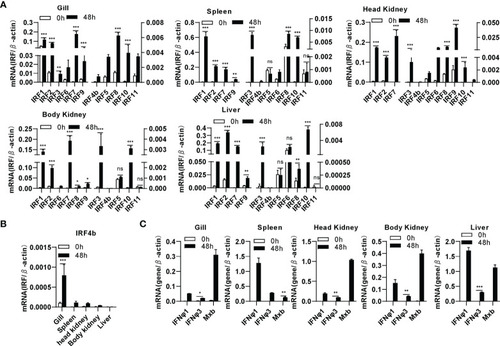
Zebrafish IRF family genes are transcriptionally induced in zebrafish tissues by SVCV infection. zebrafish adults were intraperitoneally injected with SVCV virus of 1×107 TCID50/ml (25μL/fish). 48h later, five tissues, including gill, spleen, head kidney, body kidney, and liver, were sampled for extraction of total RNAs followed by qRT-PCR analyses of 11 zebrafish IRF family genes |
|
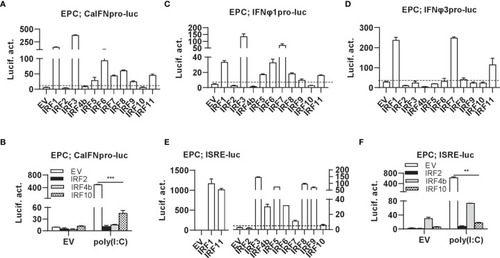
Zebrafish IRF proteins are capable to regulate fish IFN response. |
|
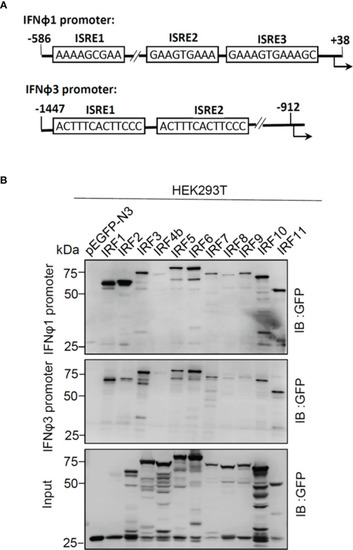
Zebrafish IRF proteins bind to zebrafish promoter DNA. |
|
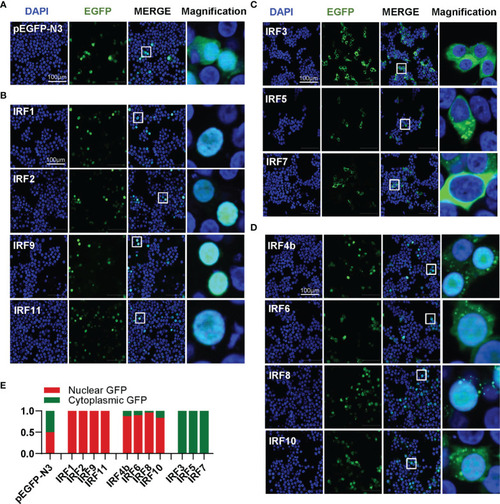
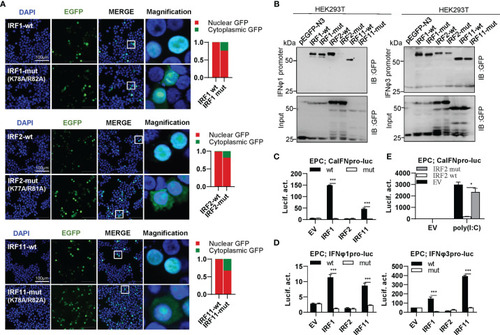
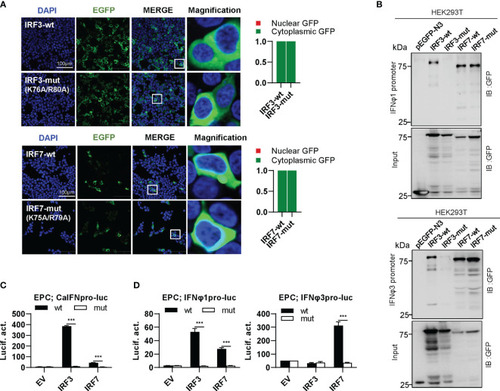
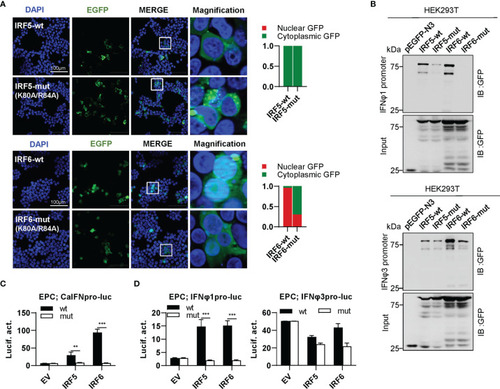
Zebrafish IRF family members show three patterns of constitutively subcellular localization. |
|
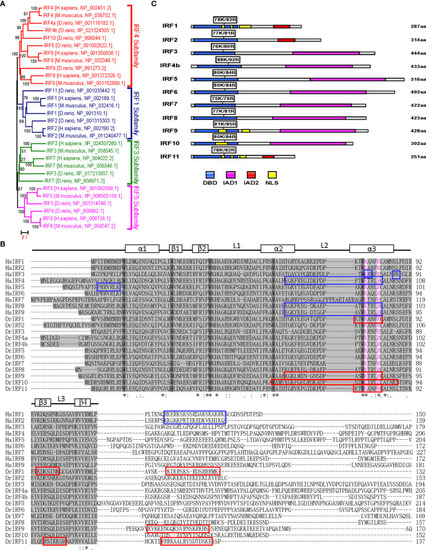
Figure 5 K78 and R82 of α3 helices in DBD domain of IRF11 are conserved across IRF family members. (A) Phylogenetic tree analysis of zebrafish and human IRF family members showing four IRF subfamilies. The phylogenetic tree was constructed by a neighbor-joining method in MEGA 5.0. The bootstrap confidence values shown at the nodes are based on 1000 bootstrap replications. (B) Multiple alignments of zebrafish IRF proteins showing a highly conserved DBD and the distribution of NLS motifs identified previously. DBD domains are gray with five conserved tryptophans. The symbols, including α-helices, β-strands and loops, indicates the secondary structures of DBD, which are marked by rectangle and line. Based on publications, the identified NLS motifs of zebrafish IRF1/10/11 are indicated by red boxes, and the NLS motifs of human IRF1/2/3/4/5/8/9 in blue boxes. Zebrafish IRF11 has a tripartite NLS motif composed of NLS1, NLS2 and NLS3, with the conserved K78 and R82 that are highlighted in purple. Identical (*) and similar (: or.) amino acid residues are indicated. (C) Schematic diagram of zebrafish IRF proteins, showing the position of two conserved basic residues corresponding to K78 and R82 of zebrafish IRF11. All mutants of zebrafish IRF protein were generated by combined mutation of the corresponding two residues to alanine. |
|
The two basic residues are essential for IRF1/2/11 to regulate IFN response. |
|
The two basic residues are essential for IRF3/7 to stimulate IFN response. |
|
The two basic residues are essential for IRF5/6 to regulate IFN response. |
|
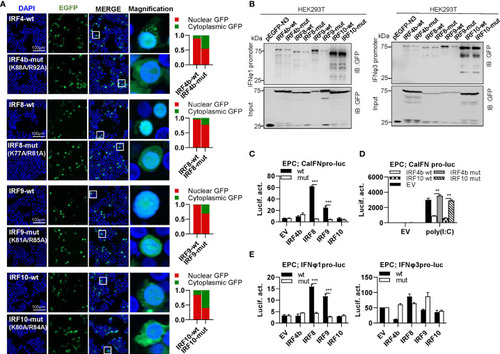
The two basic residues are essential for IRF4b/8/9/10 to regulate IFN response. |
|
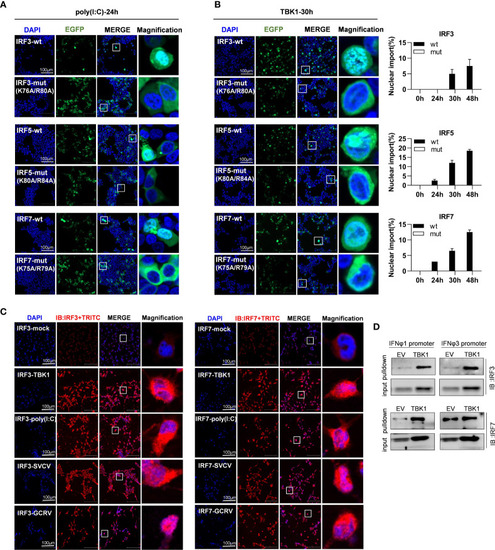
The two basic residues are essential for inducible nuclear import of IRF3/5/7 by transfection of poly(I:C) |