- Title
-
The evolutionary conserved TLDc domain defines a new class of (H+)V-ATPase interacting proteins
- Authors
- Eaton, A.F., Brown, D., Merkulova, M.
- Source
- Full text @ Sci. Rep.
|
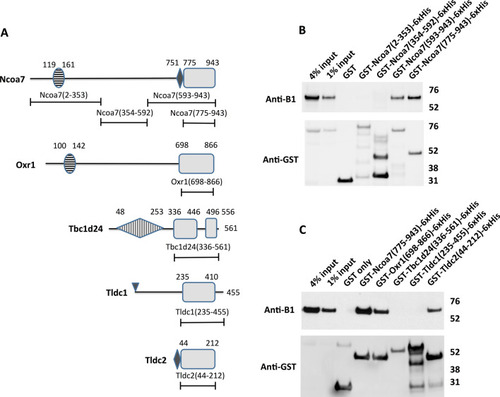
The TLDc domain is sufficient to mediate interaction between TLDc protein family members Ncoa7, Oxr1 and Tldc2 and the kidney-specific B1 subunit of V-ATPase in GST pull-down assay. ( |
|
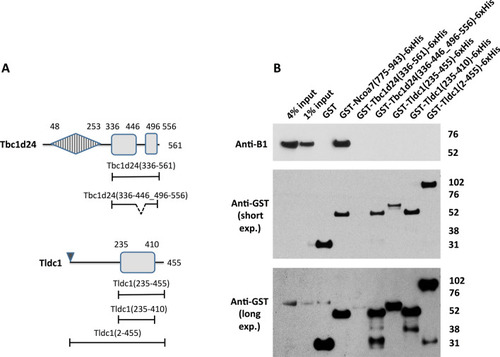
Deletion of a non-conserved loop in the TLDc domain of Tbc1d24 and the C-terminal extensions of both Tbc1d24 and Tldc1, does not result in their interaction with V-ATPase in GST pull down assay. The GST-tagged full-length purified recombinant Tldc1 (2–455) does not interact with V-ATPase in GST pull-down assay. (A) Schematic representation of the domain architecture of Tbc1d24 and Tldc1 proteins and the constructs used to study the role of the non-conserved insertion in Tbc1d24 and the C-terminal extensions in both Tbc1d24 and Tldc1 in their interaction with V-ATPase. Boundaries of the domains, non-conserved regions and constructs are indicated as in Fig. 1. (B) Anti-B1 and anti-GST western blots of a representative GST pull-down assay, using the purified GST-tagged TLDc domains of Tbc1d24 (336–561) and Tldc1 (235–455), as well as, the GST-tagged truncated versions of the TLDc domains of Tbc1d24 and Tldc1: Tbc1d24 (336-446_496-556) and Tldc1 (235–410), and the GST-tagged full-length recombinant Tldc1 (2–455) with kidney lysate as the source of the B1 subunit of V-ATPase. GST only pull-down was used as a negative control; pull-down with the GST-tagged TLDc domain of Ncoa7 (775–943) was used as a positive control, anti-GST blot was used as a loading control for comparison between samples. This experiment was repeated three times with similar results. Longer exposure of anti-GST blot is shown to confirm the presence of a relatively low amount of GST-tagged Tbc1d24 (336–561) in the pull-down assay. |
|
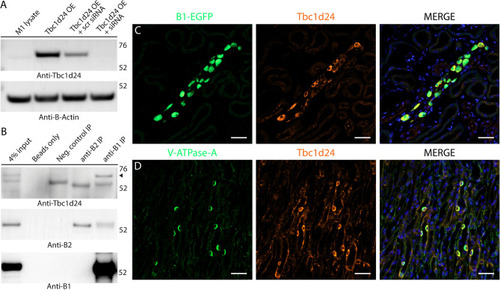
Tbc1d24 is expressed in mouse kidney intercalated cells (ICs) and co-immunoprecipitates with the kidney-specific B1 subunit of V-ATPase. ( |
|
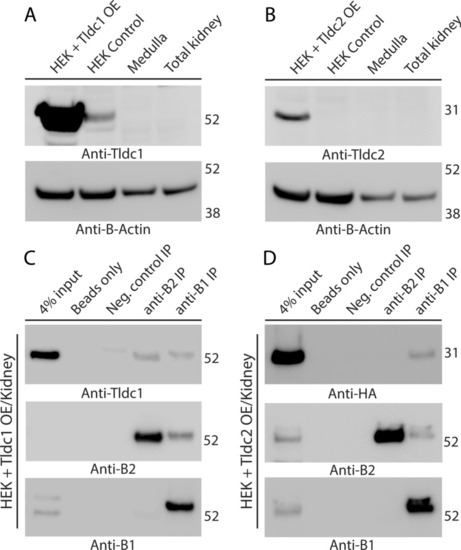
Tldc1 and Tldc2, overexpressed (OE) in HEK293T cells, co-immunoprecipitate with the B1 or B2 subunit of kidney V-ATPase. ( |
|
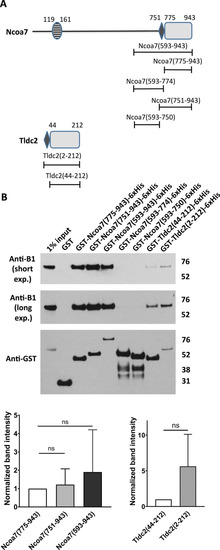
A poly-E rich motif, located upstream of the TLDc domain in Ncoa7 and Tldc2, enhances their interaction with the V-ATPase, but is not sufficient to produce significant interaction with the V-ATPase by itself. ( |
|
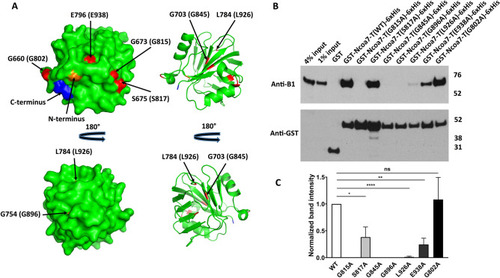
Alanine mutations of the evolutionarily conserved glycines, G815, G845 and G896, completely disrupt Ncoa7 TLDc domain interaction with the V-ATPase, while S817, L926 and E938 mutations show only partial disruption. Mutation of the non-conserved G802 residue (serving as a control) does not inhibit interaction. ( |