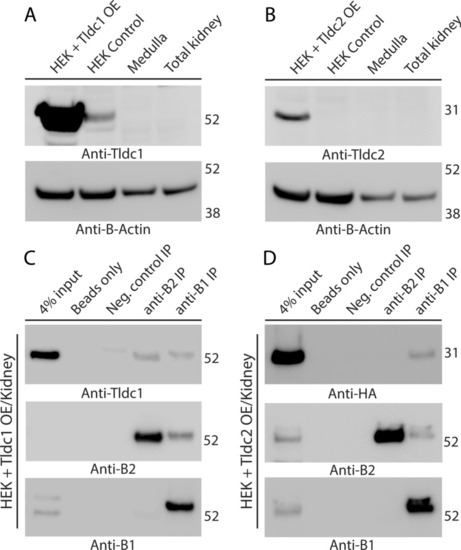
Tldc1 and Tldc2, overexpressed (OE) in HEK293T cells, co-immunoprecipitate with the B1 or B2 subunit of kidney V-ATPase. (A) Validation of the commercial anti-Tldc1 antibodies used in this study. Anti-Tldc1 western blot demonstrates that the strong band of the expected 51-kDa molecular mass is present in the lane containing HEK293T lysate from cells overexpressing mouse Tldc1 (HEK + Tldc1 OE), a much weaker band of apparently endogenous Tldc1 is present in the lane containing lysate from untransfected HEK293T cells (HEK control). The expected band of 51-kDa is not detectable in lanes containing total or medullary mouse kidney lysates. (B) Validation of the commercial anti-Tldc2 antibodies used in this study. Anti-Tldc2 western blot demonstrates that the strong band of the expected 24-kDa molecular mass is present in the lane containing lysate from HEK293T cells overexpressing mouse Tldc2 (HEK + Tldc2 OE), but the expected band of 24-kDa is not detectable in the lane containing lysate from untransfected HEK293T cells (HEK control), nor in total or medullary mouse kidney lysates. In both panels (A) and (B) anti-β-actin blot was used as a loading control. (C) Tldc1 co-immunoprecipitates with both the B1 and B2 subunit of the V-ATPase. Proteins were co-immunoprecipitated using anti-B1 and anti-B2 antibodies from mixed mouse kidney lysate and HEK293T + Tldc1 overexpressing lysate and then analyzed by western blot, using anti-Tldc1 antibodies. The specific band of the expected 51 kDa molecular mass is present in both the anti-B1 immunoprecipitation (anti-B1 IP) and anti-B2 immunoprecipitation (anti-B2 IP) lanes. This experiment was repeated three times with similar results. (D) Tldc2 co-immunoprecipitates with the kidney-enriched B1 subunit of V-ATPase (V-ATPase), but not with the ubiquitously expressed B2 subunit of V-ATPase. Proteins were co-immunoprecipitated using anti-B1 and anti-B2 antibodies from mixed mouse kidney lysate and HEK293T + Tldc2 overexpressing lysate and then analyzed by western blot, using HRP-conjugated anti-HA antibodies, which recognize the HA-tagged overexpressed Tldc2 protein. The specific band of the expected 24 kDa molecular mass is present only in the anti-B1 immunoprecipitation (anti-B1 IP) lane. In both panels (C) and (D) anti-B1 and anti-B2 western blots are shown to confirm the successful immunoprecipitation of B1 and B2 subunits of V-ATPase. This experiment was repeated three times with similar results.
|

