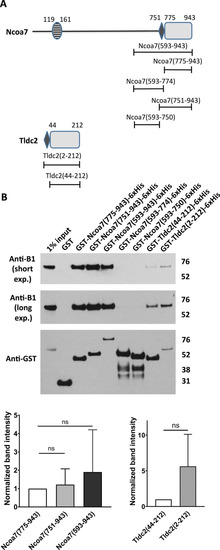
A poly-E rich motif, located upstream of the TLDc domain in Ncoa7 and Tldc2, enhances their interaction with the V-ATPase, but is not sufficient to produce significant interaction with the V-ATPase by itself. (A) Schematic representation of the domain architecture of the Ncoa7 and Tldc2 proteins and the constructs used to study the role of the poly-E rich motifs from Ncoa7 and Tldc2 in their interaction with V-ATPase. The conserved regions of the TLDc domains are shown as grey rectangles and poly-E rich motifs as small black rhombuses. Other details and boundaries of the domains and constructs are as indicated in Fig. 1. (B) Anti-B1 and anti-GST western blots of a representative GST pull-down assay, using the purified GST-tagged TLDc domains of Ncoa7 (775–943) and Tldc2 (44–212) without poly-E rich motifs, Ncoa7 (751–943), Ncoa7 (593–943) and Tldc2 (2–212) proteins containing both the poly-E rich motif and the TLDc domain, Ncoa7 (593–750) lacking both the poly-E rich motif and the TLDc domain, and finally Ncoa7(593–774), containing the poly-E rich motif but lacking the TLDc domain. A longer exposure of anti-B1 blot is shown to better visualize a relatively weaker ~ 55 kDa band of B1 subunit of V-ATPase in Tldc2 (44–212) and Tldc2 (2–212) GST pull-downs. GST only pull-down was used as a negative control; anti-GST blot was used as a loading control for comparison between samples. This experiment was repeated three times with similar results. (C) Quantification of western blotting results by band densitometry analysis. Anti-B1 band densities were divided by anti-GST band densities and then normalized relative to the Ncoa7 (775–943) B1/GST ratio for all Ncoa7 constructs or relative to Tldc2 (44–212) B1/GST ratio for Tldc2 constructs. All values are means ± SE. ns—non significant, P = 0.6852 for Ncoa7 (775–943) vs. Ncoa7 (751–943), P = 0.5116 for Ncoa7 (775–943) vs. Ncoa7 (593–943), P = 0.3611 for Tldc2 (44–212) vs. Tldc2 (2–212), by t-test. Note, that Ncoa7 (751–943), Ncoa7 (593–943) and Tldc2 (2–212) constructs with both poly-E rich motif and TLDc domain show a trend toward more efficient pulling down the B1 subunit of V-ATPase in comparison with the TLDc only domains of Ncoa7 (775–943) and Tldc2 (44–212). However, the Ncoa7 construct (593–774) containing the poly-E rich motif but lacking the TLDc domain did not pull down the B1 subunit, at all.
|

