- Title
-
Rab5ab-Mediated Yolk Cell Membrane Endocytosis Is Essential for Zebrafish Epiboly and Mechanical Equilibrium During Gastrulation
- Authors
- Marsal, M., Hernández-Vega, A., Pouille, P.A., Martin-Blanco, E.
- Source
- Full text @ Front Cell Dev Biol
|
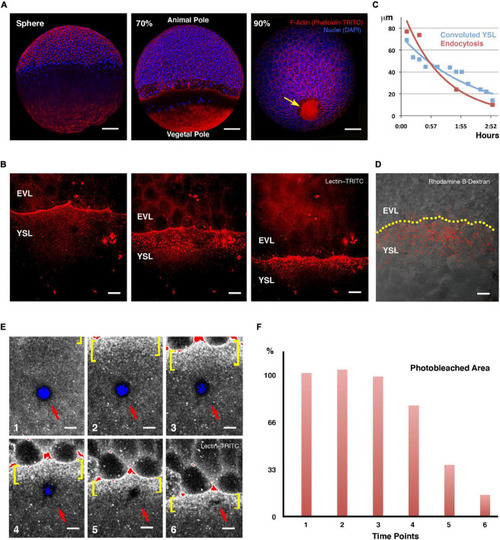
Yolk cell membrane endocytosis at the E-YSL. |
|
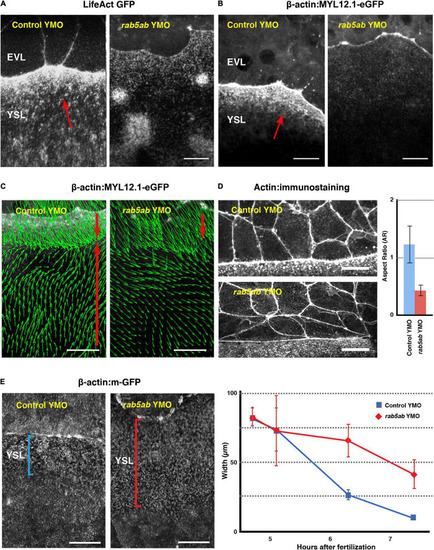
Endocytosis and Epiboly are impaired after |
|
Cytoskeleton dynamics and EVL leading cells shapes are affected by |
|
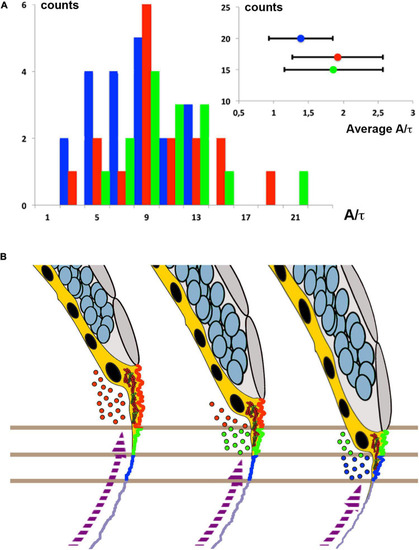
Biomechanics of yolk cell endocytosis impaired embryos. |
|
Membrane cortical tension and endocytosis at the E-YSL are necessary for epiboly progression. |