- Title
-
Transient, flexible gene editing in zebrafish neutrophils and macrophages for determination of cell-autonomous functions
- Authors
- Isiaku, A.I., Zhang, Z., Pazhakh, V., Manley, H.R., Thompson, E.R., Fox, L.C., Yerneni, S., Blombery, P., Lieschke, G.J.
- Source
- Full text @ Dis. Model. Mech.
|
|
|
|
|
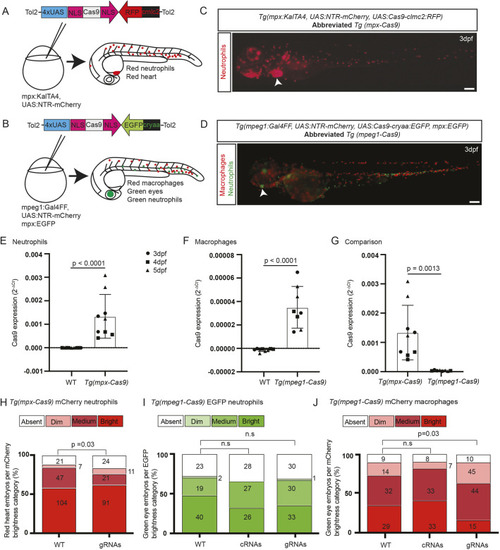
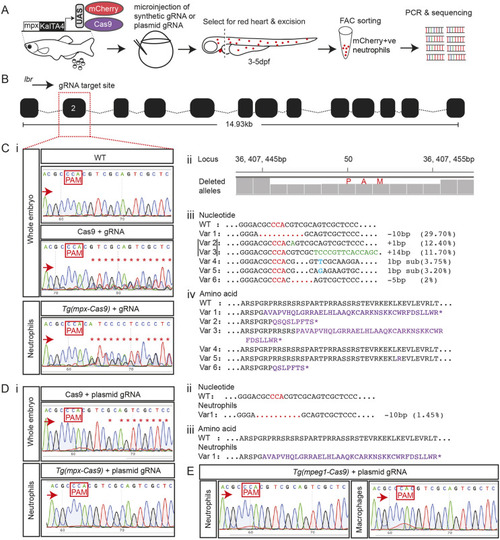
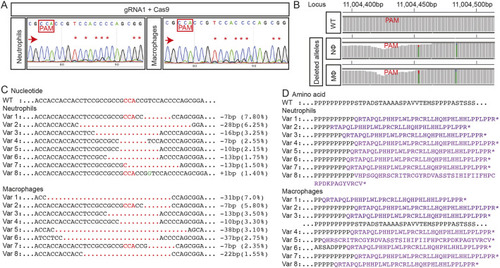
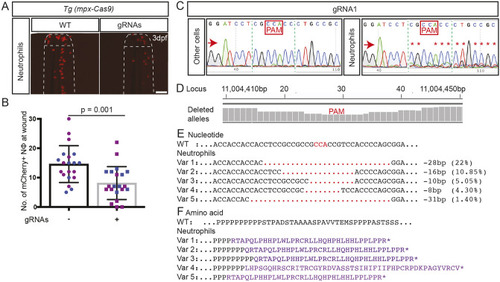
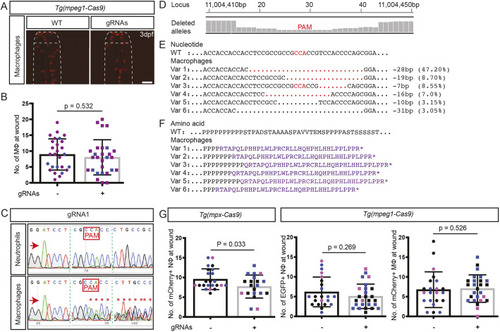
(A) Zebrafish trim33 locus showing target sites for two gRNAs in exon 1 and 15. (B) Sanger chromatogram of WT whole-embryo DNA (upper row) compared to F0 Tg(mpeg1:Gal4FF/UAS:NTR-mCherry)(mpx:GFP) crispant embryos injected with two multiplexed trim33 gRNAs complexed to exogenous Cas9 protein (lower row). (C) Fluorescent images of GFP-labelled neutrophils and mCherry-labelled macrophages at 3.5 h after caudal fin transection, in WT and trim33 crispant 3 dpf Tg(mpeg1:Gal4FF/UAS:NTR-mCherry)(mpx:GFP) embryos. Embryos from the same experiment as in B. (D) Neutrophil and macrophage numbers at wound site at 3.5 h post-injury. Red arrows indicate the sequencing direction; red asterisks indicate sequence heterogeneity due to on-target gene editing; green vertical dashed lines indicate cropped areas of the chromatogram; PAM, protospacer adjacent motif highlighted in red boxes. Unpaired two-tailed Student’s t-test (P<0.0001) of pooled data from two independent experiments indicated by different colours. Scale bars: 100 µm. |
|
|
|
|
|
|