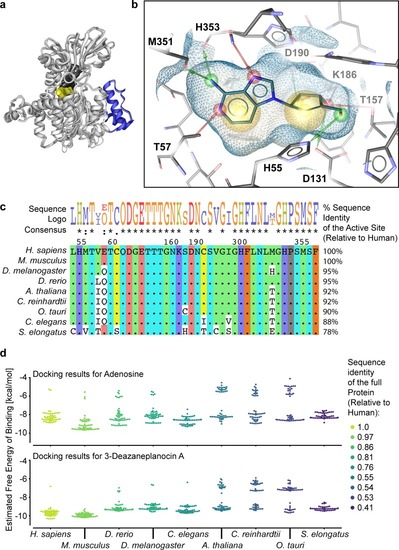
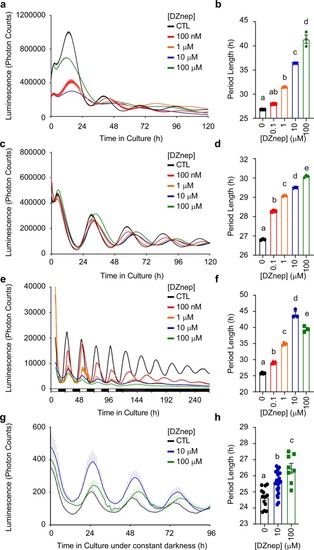
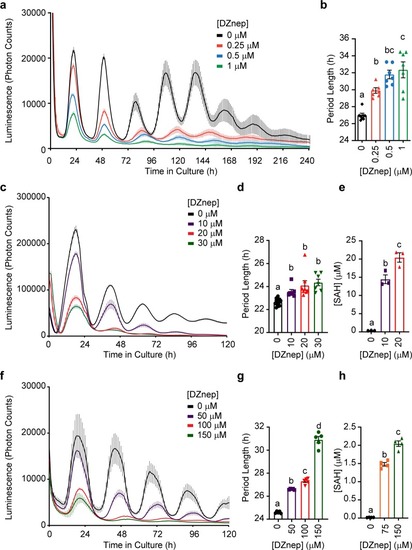
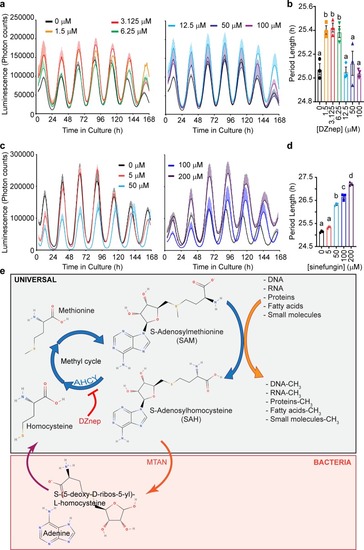
|
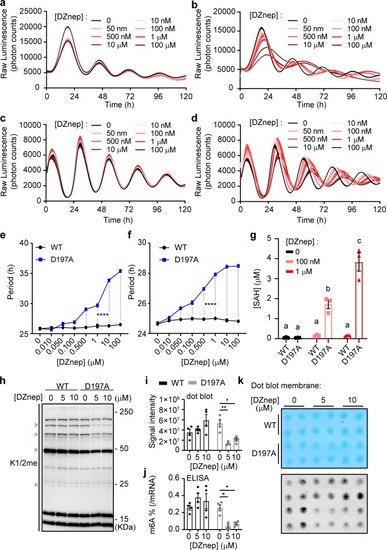
Rewiring the methyl cycle protects mammalian cells from methylation deficiency.a, b Mean luminescence ± SEM of human Bmal1-luc U-2 OS cells transfected with WT and mutant MTAN, respectively, and treated with different concentrations of DZnep, n = 3 dishes per treatment. c, d Mean luminescence ± SEM of PER2::LUC MEFs transfected with WT and mutant MTAN, respectively, and treated with different concentrations of DZnep, n = 3 dishes per treatment. e, f Mean period ± SEM of Bmal1-luc U-2 OS cells and PER2::LUC MEFs from a, b and c, d, respectively, analyzed by two-way ANOVA followed by Bonferroni’s test, n = 3 dishes per treatment. The gray bars indicate which comparisons reach significance, all p < 0.0001 (****). g Mean SAH concentration ± SEM in DZnep-treated PER2::LUC MEFs, n = 3 dishes per treatment, analyzed by Two-Way ANOVA (all sources of variations p < 0.0001) followed by Bonferroni’s test; all indicated comparisons (a vs. b vs. c) at least p < 0.05. See also Supplementary Fig. 6. h Immunoblots using an antibody against mono- and di- methylated lysine (K1/2me) reveal that the methylation of some proteins is inhibited by DZnep, and rescued by WT but not D197A MTAN. The red > signs indicate such proteins. Actin loading control is shown in Supplementary Fig. 6i. i, j Mean mRNA m6A methylation levels ± SEM in PER2::LUC MEFs transfected with WT or D197A MTAN, then treated with DZnep for 48 h; i, j showing dot blot assay and enzyme-linked immunosorbent assay-based quantification (ELISA), respectively. Dot blot and ELISA analyzed separately by Two-Way ANOVA (DZnep treatment, MTAN effect and interactions at least p < 0.05) followed by Bonferroni’s test (*p < 0.05; **p < 0.01), n = 4 dishes. k Dot blot membrane quantified in i, with 150 ng mRNA/dot, first stained with methylene blue (top) as a loading control.
|