- Title
-
Macrophages Respond Rapidly to Ototoxic Injury of Lateral Line Hair Cells but Are Not Required for Hair Cell Regeneration
- Authors
- Warchol, M.E., Schrader, A., Sheets, L.
- Source
- Full text @ Front. Cell. Neurosci.
|
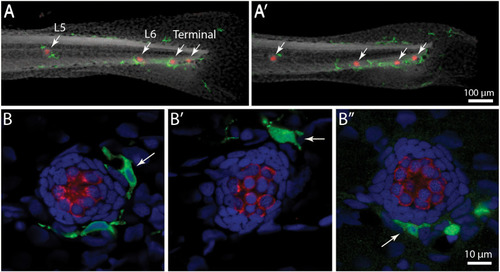
Distribution of macrophages in the posterior lateral line of larval zebrafish. |
|
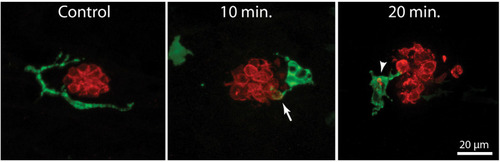
Macrophage response to neomycin ototoxicity. Images are maximum-intensity projections of confocal |
|
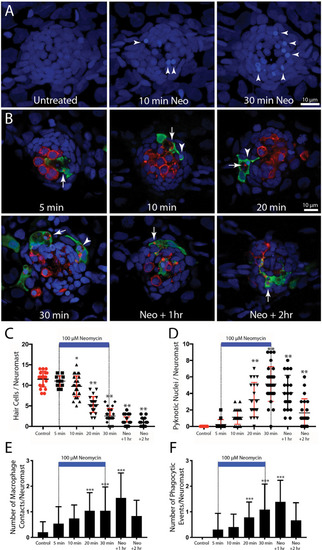
Detailed time course of macrophage response to neomycin ototoxicity. Fish (6 dpf) were incubated in 100 μM neomycin. Some fish were fixed after 5, 10, 20, or 30 min of neomycin treatment, while other fish were removed from neomycin after 30 min, and rinsed and maintained in fresh embryo medium (EM) for 1 or 2 h. Fixed specimens were processed for immunolabeling of YFP-expressing macrophages (green) and hair cells (otoferlin, red), and nuclei were labeled with DAPI (blue). Images in panel (A) are maximum intensity projections, while images in panel (B) are single z-sections taken from 15 μm-depth confocal stacks. (A) Formation of pyknotic nuclei in response to neomycin treatment. Images show DAPI labeling in an untreated neuromast and in neuromasts that were exposed to neomycin for 10 and 30 min. Neomycin induces the formation of pyknotic nuclei (arrowheads), which are assumed to be dying hair cells. (B) Single z-stack sections at various time intervals after initiation of neomycin treatment. Arrows in all images indicate evidence for macrophage phagocytosis of dying hair cells. A few specimens displayed a macrophage response after only 5 min of neomycin exposure. Note that the macrophage in the 5 min image has internalized otoferlin-labeled debris (arrow). At later time points, macrophages had engulfed both otoferlin-labeled debris as well as pyknotic nuclei (arrowheads in 10, 20, and 30 min images). Also, beginning at 20 min of neomycin exposure, the number of otoferlin-labeled hair cells was significantly reduced. Neuromasts at 30 min exposure and 1 and 2 h recovery typically contained 0–3 surviving hair cells. (C) Surviving hair cells as a function of exposure/post-exposure time. Intact hair cells were identified by healthy nuclei that were completely enclosed by the otoferlin-labeled hair cell membrane. A significant decrease in hair cell numbers was observed, beginning after 10 min of neomycin exposure (***p = 0.019, **p < 0.0001, Tukey’s multiple comparisons test). (D) Changes in the numbers of pyknotic nuclei plotted as a function of neomycin exposure/post-exposure time. The number of pyknotic nuclei/neuromast became significantly elevated after 20 min of neomycin exposure (**p < 0.0001). The time course of the neomycin-induced formation of pyknotic nuclei parallels the death of hair cells. (E) Contacts between macrophages and hair cells increased after 20 min of neomycin treatment (***p = 0.0038, one-way ANOVA) and remained elevated until 60 min after treatment. (F) Macrophage phagocytosis of hair cell debris (i.e., internalization of otoferlin-labeled material and/or pyknotic nuclei) was also increased, beginning after 20 min of neomycin treatment (***p < 0.0044, one-way ANOVA). |
|
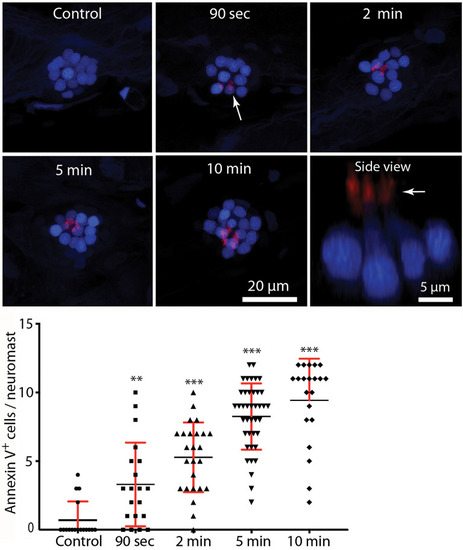
Rapid externalization of phosphatidylserine (PtS) in response to neomycin treatment. Larval zebrafish (6 dpf) were incubated in Alexa 555-conjugated annexin V and neomycin was added to the water, for a final concentration of 100 μM. Fish were euthanized and fixed after 90 s, 2, 5, or 10 min of exposure to neomycin. Annexin V labeling was observed on the stereocilia bundles of most neomycin-treated fish (e.g., arrow, 90-s image, each red structure represents a single stereocilia bundle), and indicates the presence of PtS on the outer membrane surface. We observed annexin V binding as early as 90 s after the initiation of neomycin treatment (** |
|
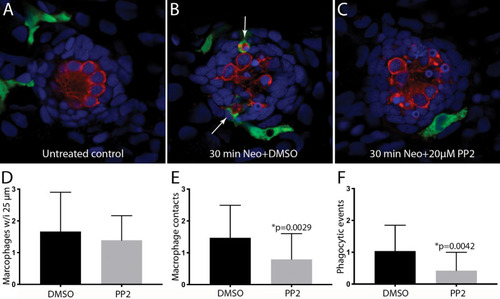
Inhibition of Src-family kinases reduced macrophage entry into injured neuromasts. Larval zebrafish were treated in 20 μM PP2, an inhibitor of several Src kinases. Control fish were treated in parallel with 0.1% DMSO. After 1 h pretreatment, neomycin was added to the water of both treatment groups (for a final concentration of 100 μM), and all specimens were euthanized and fixed after 30 min of neomycin exposure. |
|
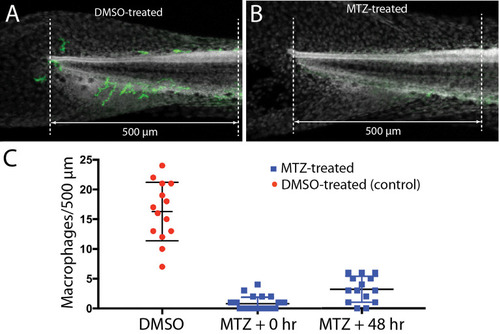
Selective depletion of macrophages in |
|
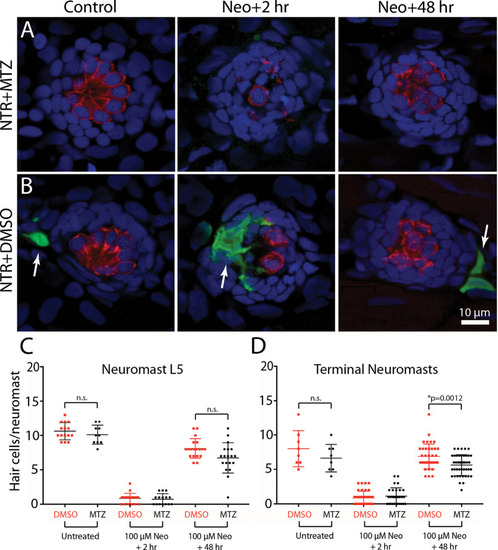
Depletion of macrophages had minimal impact on hair cell regeneration. |