- Title
-
Suppressive effects of valproic acid on caudal fin regeneration in adult zebrafish
- Authors
- Lee, Y., Kim, D., Lee, C.J.
- Source
- Full text @ Animal Cells Syst (Seoul)
|
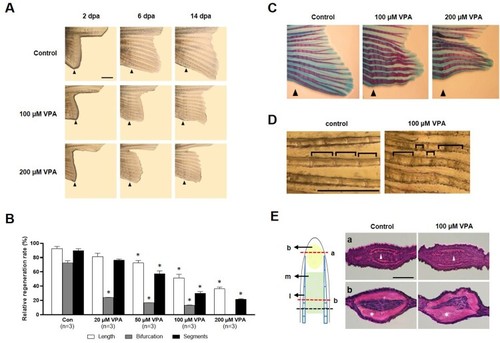
Suppression of caudal fin regeneration after amputation in VPA-treated zebrafish. (A) Images show the regenerated fin in each group at 2, 6, and 14 dpa. Arrowheads indicates the amputation site. Scale bar, 2 mm. (B) Bars indicate the regeneration ratio of length, segments, and bifurcating ray at 14 dpa. Data were expressed as the means ± S.E.M ( |
|
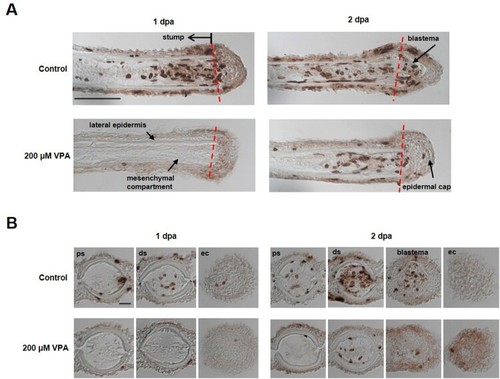
Delay of blastema formation in the regenerated fin by treatment with 200 µM VPA. (A) Images show the sagittal sections of the regenerated fin using BrdU staining at 1 and 2 dpa. A red dotted line, the amputation site. Scale bar, 500 µm. BrdU-labeled cells in the fin of control zebrafish were shown in the mesenchymal compartment, lateral epidermis and epidermal cap at 1 dpa, but not in 200 µM VPA-treated zebrafish. No blastema was formed in the regenerated area of 200 µM VPA-treated zebrafish. (B) At 1 dpa, BrdU-labeled cells were detected in the proximal stump (ps), distal stump (ds), and regenerated epidermal cap (ec) in the control, but hardly detected in 200 µM VPA-treated zebrafish. At 2 dpa, A few BrdU-labeled cells were detected in the distal stump of 200 µM VPA-treated zebrafish. Scale bar, 200 µm. |
|
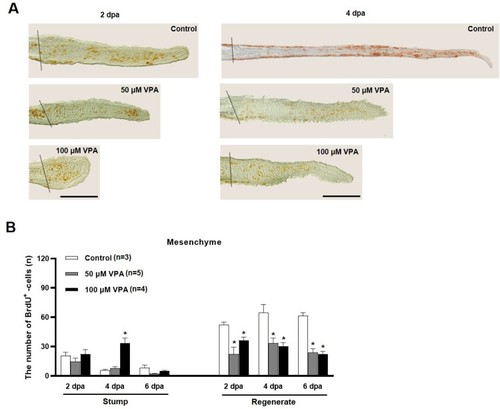
Quantification of BrdU-labeled cells in the mesenchymal area of regenerated fin at 2, 4, and 6 dpa. (A) Images show BrdU-labeled cells in the sagittal sections at 2 and 4 dpa. A dotted line indicates the amputation site, and brown colored dots are BrdU-labeled cells. Scale bar = 500 µm. (B) Bars indicate the number of BrdU-labeled cells in mesenchymal area of the stump and regenerated fin at 2, 4, and 6 dpa. Data were expressed as the means ± S.E.M (n≥3) and tested via |
|
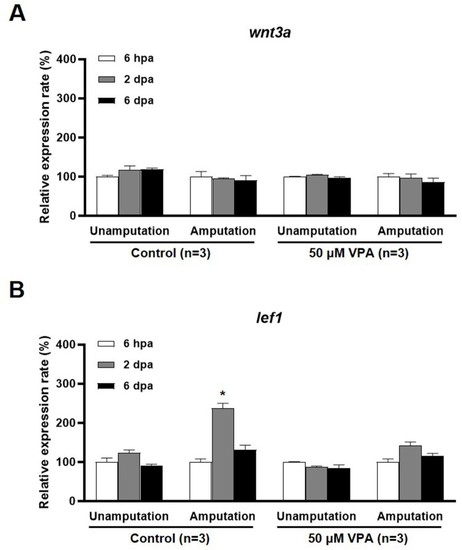
qRT-PCR analysis of |
|
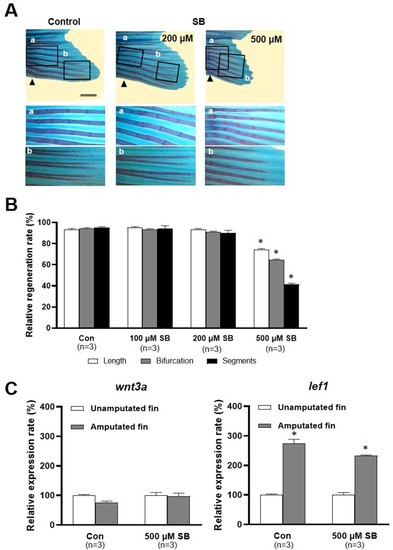
Effects of sodium butyrate on caudal fin regeneration of adult zebrafish. (A) Images show Alcian blue and alizarin red staining of the regenerated fin in the control and SB (200 and 500 µM)-treated zebrafish at 14 dpa. Arrowheads, the amputation site. Scale bar, 2 mm. (B) Bars indicate the regeneration ratio of length, segments, and bifurcating ray at 14 dpa. Decreased regeneration rate was shown in only 500 µM SB-treated zebrafish. Data were expressed as the means ± S.E.M ( |