- Title
-
Neurons Expressing Pathological Tau Protein Trigger Dramatic Changes in Microglial Morphology and Dynamics
- Authors
- Hassan-Abdi, R., Brenet, A., Bennis, M., Yanicostas, C., Soussi-Yanicostas, N.
- Source
- Full text @ Front. Neurosci.
|
Microglia displays dramatic changes in morphology and dynamics in the presence of hTauP301L-expressing neurons. |
|
Genetic depletion of microglia worsens the pathology in Tg(HuC-hTauP301L:DsRed) embryos. |
|
Microglia phagocytic activity is increased in presence of hTauP301L-expressing, but appears non-sufficient in eliminating all apoptotic neurons. PHENOTYPE:
|
|
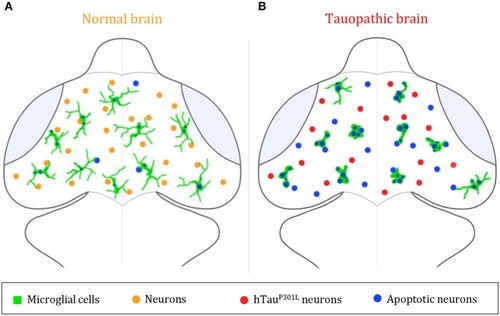
Summary illustration. |