- Title
-
Wilms Tumor 1b Expression Defines a Pro-regenerative Macrophage Subtype and Is Required for Organ Regeneration in the Zebrafish
- Authors
- Sanz-Morejón, A., García-Redondo, A.B., Reuter, H., Marques, I.J., Bates, T., Galardi-Castilla, M., Große, A., Manig, S., Langa, X., Ernst, A., Piragyte, I., Botos, M.A., González-Rosa, J.M., Ruiz-Ortega, M., Briones, A.M., Salaices, M., Englert, C., Mercader, N.
- Source
- Full text @ Cell Rep.
|
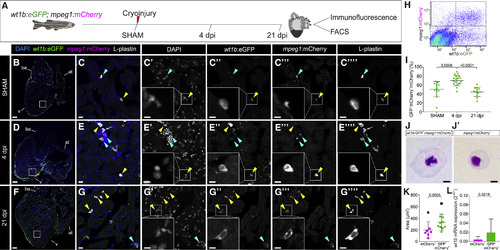
wt1b:eGFP Expression Defines a Population of mpeg1:mCherry+ Cells in the Zebrafish Heart (A) Tg(wt1b:eGFP;mpeg1:mCherry) adult zebrafish were cryoinjured and hearts were collected to perform FACS or immunofluorescence (IF) staining. (B–G’’’’) IF on heart sections. The yellow arrowheads indicate eGFP+;mCherry+;L-plastin+ cells, and the blue arrowheads indicate mCherry+;L-plastin+ cells. (B), (D), and (F) are whole-heart views, remaining panels are zoomed views showing merged (C, E, and G) or single channels. Representative images from sham (n = 7), 4 dpi (n = 14), and 21 dpi (n = 8) processed hearts from 2 experimental replicates are shown. (H) Representative scatterplot of FACS-sorted cells from Tg(wt1b:eGFP;mpeg1:mCherry) hearts at 4 dpi. (I) Quantification of flow cytometry data showing the percentage of eGFP+;mCherry+/mCherry+ cells at different time points. Statistical significance is calculated by one-way ANOVA, followed by Tukey’s multiple comparisons test. Two experimental replicates are shown. (J and J’) May-Grünwald and Giemsa histological staining of mCherry+ and eGFP+;mCherry+ cells isolated from hearts at 4 dpi. Shown are representative examples of 9/10 and 6/8 analyzed cells, for double- and single-positive cells, respectively. Two experimental replicates are shown. (K) Area measurements of sorted cells from (J) and (J’). Means ± SDs are shown. Calculations were done using Welch’s t test. The black points represent statistically significant outliers, by the Grubbs test (α = 0.05), excluded from statistical analysis. (L) qRT-PCR for wt1b in mCherry+ and eGFP+;mCherry+ cells isolated from cryoinjured hearts. The points represent biological replicates. Means ± SDs are shown; two-tailed unpaired t test. Ten experimental replicates are shown. Scale bars, 100 μm (B, D, and F), 10 μm (C, E, G, J, and J’), and 5 μm (magnified views in C’–G’’’). at, atrium; ba, bulbus arteriosus; dpi, days post-injury; FACS, fluorescence-activated cell sorting; v, ventricle. EXPRESSION / LABELING:
|
|
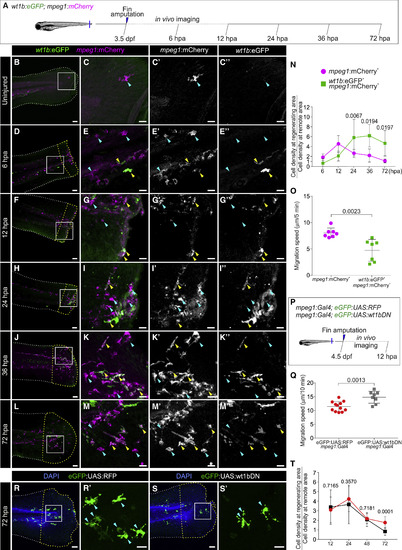
wt1b:eGFP+ Macrophages Home to the Site of Injury during Caudal Fin Regeneration, and Wt1b Regulates Their Migratory Behavior (A) Caudal fins from Tg(wt1b:eGFP;mpeg1:mCherry) zebrafish larvae were amputated at 3.5 dpf and either fixed at different time points and processed for IF or embedded for in vivo imaging. (B–M’’) Whole-mount IF on caudal fins. Merged and single eGFP and mCherry channels of the magnified views from boxed areas are shown on the right panels. The yellow arrowheads point to double-positive cells, and the blue arrowheads to single mCherry+ cells. The white and yellow dotted lines outline the remote and regenerating areas, respectively. The regenerating area is defined as 100 μm distal from the amputation plane until the fin tip. Maximum intensity projections are shown. Also shown are representative images from 6 hpa (n = 7), 12 hpa (n = 6), 24 hpa (n = 8), 36 hpa (n = 4), and 72 hpa (n = 5) samples from 2 experimental replicates. (N) Accumulation index of eGFP+;mCherry+ or mCherry+macrophages at the regenerating area of animals from (D)–(M’’). Calculated as cell density at the regenerating area/cell density at the remote area. Two-way ANOVA, followed by Sidak’s post hoc test. (O) Quantification of the migration speed of eGFP+;mCherry+ versus mCherry+ macrophages. The dots indicate mean values for macrophages counted in n = 7 larvae from 3 experimental replicates. Means ± SDs are shown; two-tailed unpaired t test. (P) Analysis of migratory behavior of macrophages during the first 12 hpa upon Wt1b inhibition by expressing a dominant-negative isoform in macrophages using the Gal4;UAS system. (Q) Quantification of macrophage migration speed. Means ± SDs are shown. Individual points represent the average migration of all macrophages per embryo from 3 experimental replicates. Two-tailed unpaired Student’s t test. (R–S’) Whole-mount IF on caudal fins from Tg(eGFP:UAS:RFP) (R) and Tg(eGFP:UAS:wt1bDN) (S) lines, both in Tg(mpeg1:Gal4)background. (R’) and (S’) are magnified views of boxed areas in (R) and (S), respectively; arrowheads point to eGFP+ macrophages. The white and yellow dotted lines outline the remote and regenerating areas, respectively. The regenerating area is defined as 100 μm distal from the amputation plane until the fin tip. The maximum intensity projections are shown. (T) Accumulation index of eGFP:UAS:RFP or eGFP:UAS:wt1bDN macrophages at the regenerating area of animals from (R)–(S’) at 12, 24, 48, and 72 hpa, calculated as in (N). An average of n = 12 embryos was analyzed per time point; two-tailed unpaired t test. Scale bars, 50 μm (B, D, F, H, J, L, R, and S) and 20 μm (magnified views). dpf, days post-fertilization; hpa, hours post-amputation. EXPRESSION / LABELING:
PHENOTYPE:
|
|
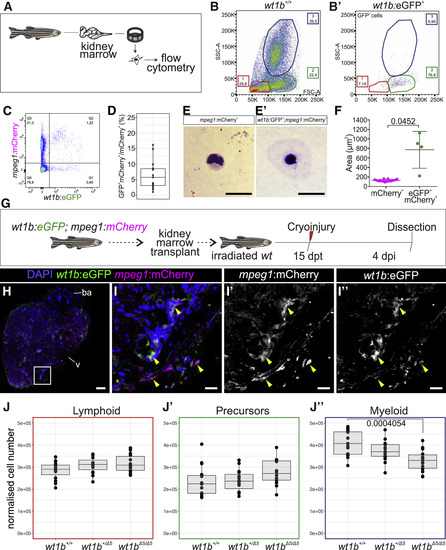
wt1b:eGFP+ Cells Are Present in the Hematopoietic Niche and Can Contribute to Cardiac Macrophages in the Regenerating Heart (A) Whole kidney marrow (WKM) cells from different transgenic or mutant lines were isolated and analyzed by flow cytometry. (B and B’) Forward scatter (FSC-A) versus side scatter (SSC-A) plot of WKM cells. (B) WKM cells of wild-type adult zebrafish gating into 3 distinct populations (gate 1, lymphocytes; gate 2, progenitors; gate 3, myeloid cells). Shown is the percentage of each population normalized to all single alive cells. Erythrocytes were removed from the FACS plot to facilitate data visualization (representative plot of 5 replicates, B, and 2 replicates, B’). (B’) eGFP+ cells from the Tg(wt1b:eGFP) WKM are enriched in gate 2. (C) Example plot of FACS purified cells from Tg(wt1b:eGFP;mpeg1:mCherry) WKM (representative plot of total n = 15 from 3 experimental replicates). (D) Quantification of the percentage of eGFP+;mCherry+/mCherry+WKM cells. Means ± SDs are shown. The points are values from individual animals (n = 15 from 3 experimental replicates) (E and E’) May-Grünwald Giemsa histological staining of mCherry+and eGFP+;mCherry+ WKM cells. A representative example from a total of 245 mCherry+ and 16 eGFP+;mCherry+ cells (2 independent experiments) is shown. (F) Cell size measurements of mCherry+ and eGFP+;mCherry+ cells. Means ± SDs are shown. The statistical analysis was performed with Welch’s t test. (G) Tg(wt1b:eGFP;mpeg1:mCherry) WKM cells were transplanted into irradiated wild-type hosts. At 15 days post-transplantation (dpt), the host’s heart was cryoinjured and fixed at 4 days post-injury (dpi). (H–I’’) IF staining of a heart section from (G). (I–I’’) are merged and single channels of the magnified view of the boxed area in (H). The yellow arrowheads indicate eGFP+;mCherry+cells. Representative examples from 3 biological replicates from 2 technical replicates are shown. (J–J’’) Composition of immune cells in the WKM of wt1b+/+, wt1b+/Δ5, and wt1bΔ5/Δ5 lines. Boxplots of normalized cell numbers of cell populations in gates 1 (J, lymphoid), 2 (J’, precursors), or 3 (J’’, myeloid) in wt1b+/+ (n = 15), wt1b+/Δ5 (n = 15), and wt1bΔ5/Δ5 (n = 14) WKM are shown. Data from 3 experimental replicates. Normalized cell numbers relate to cell numbers per 106 events of living single cells. Myeloid cell numbers (J’’) are significantly lower in wt1bΔ5/Δ5than in wt1b+/+ by one-way ANOVA, followed by Tukey’s post hoc test. Scale bars, 20 μm (E, E’, and H) and 100 μm (I–I’’). |
|
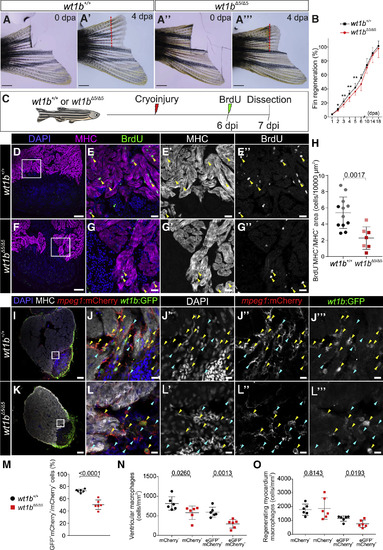
Heart and Fin Regeneration Are Impaired in wt1b Mutants (A–A’’’) Caudal fin amputation was performed to wild-type wt1b+/+and mutant wt1bΔ5/Δ5 adult fish. Fin regrowth was assessed until complete regeneration at 18 dpa. Shown are representative examples at 0 dpa (A and A’’) and 4 dpa (A’ and A’’’). The red dotted line represents the amputation plane. (B) Quantification of fin regeneration. The 3 most regrown rays were measured per animal and normalized by the initial amputated fin length. The data are from 2 independent experiments: experiment 1 (1–18 dpi), wt1b+/+ = 7 and wt1bΔ5/Δ5 = 5; experiment 2 (1–6 dpi), wt1b+/+ = 10 and wt1bΔ5/Δ5 = 10. Means ± SDs are shown; two-tailed unpaired t test (∗p < 0.05 and ∗∗p < 0.01). (C) Ventricular cryoinjury was performed on wt1b+/+ and wt1bΔ5/Δ5adult fish, and cardiomyocyte proliferation was assessed from 6 to 7 dpi by BrdU incorporation. (D–G’’) IF staining on sections of wt1b+/+ (D–E’’) and wt1bΔ5/Δ5 hearts (F–G’’). Nuclei were counterstained with DAPI. (E)–(E’’) and (G)–(G’’) are merged and single channels of boxed areas in (D) and (F), respectively. (H) Quantification of cardiomyocyte proliferation. The light- and dark-colored dots represent animals from two independent experiments. Means ± SDs are shown. Two-tailed unpaired Student’s t test. (I–O) Localization of wt1b:eGFP;mpeg1:mCherry cells in cryoinjured wt1b+/+ and mutant wt1bΔ5/Δ5 hearts at 7 dpi. (I–L’’’) IF staining at 7 dpi heart sections. Nuclei were counterstained with DAPI. Shown are whole heart sections (I and K) and magnified views of merged and single channels (J–J’’’ and L–L’’’). The yellow arrowheads indicate double-positive cells, and the blue arrows indicate mpeg1:mCherry+ cells. (M) The percentage of wt1b:eGFP+;mpeg1:mCherry+/mpeg1:mCherry+cells per heart section. (N) Quantification of the number of single- and double-positive cells per area in whole heart sections. (O) Quantification of the number of single- and double-positive cells in the regenerating myocardium defined as the area of 100 μm adjacent to the injury. In all of the graphs, each dot represents data from one heart section with the maximum injury area. n = 6 hearts analyzed per group from 1 experimental replicate. Means ± SDs are shown; two-tailed unpaired t test. Scale bars, 1 mm (A–A’’’), 100 μm (D, F, I, and K), 20 μm (E–E’’ and G–G’’), and 10 μm (J–J’’’ and L–L’’’). BrdU, 5′-bromo-2′-deoxyuridine; dpa, days post-amputation; dpi, days post-injury; MHC, myosin heavy chain. PHENOTYPE:
|
|
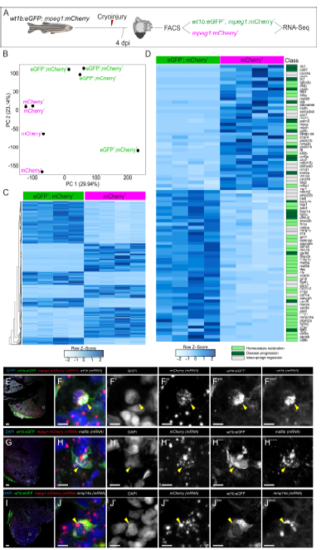
Differential gene expression of wt1b -positive macrophages. Related to Figure 2 and Table S1. A, wt1b:eGFP+;mpeg1:mCherry+ and mpeg1:mCherry+ cells were FAC-sorted from cryoinjured adult zebrafish hearts at 4 dpi and their transcriptomes analyzed using RNA-seq. B, Principal component analysis of wt1b:eGFP+; mpeg1:mCherry+ and mpeg1:mCherry+ cells. C, Heatmap indicating all significant differentially expressed genes between both populations. D, Heatmap of genes from (C) whose function has been described in macrophages. Classification of gene function in macrophages according to literature: grey, genes involved in macrophage regulation including differentiation, phagocytosis and apoptosis; light green, genes involved in homeostasis restoration; dark green, genes involved in disease progression. E-J’’’’, Validation of RNA-seq target genes by RNAScope in situ hybridization followed by anti-GFP immunostaining on cryoinjured wt1b:eGFP; mpeg1:mCherry heart sections at 4 dpi. Signal from wt1b, mafbb and mmp14a antisense riboprobes co-localizes with mCherry mRNA and eGFP signal (arrowheads). Note that large dots for mCherry channels represent background staining, and small dots correspond to signal. dpi, days post injury; FACS, fluorescenceactivated cell sorting; PC, principal component; Mφ, macrophage. Scale bars, 50 μm (E, G, I) and 5 μm (F-F’’’’, H-H’’’’ and J-J’’’’). |
|
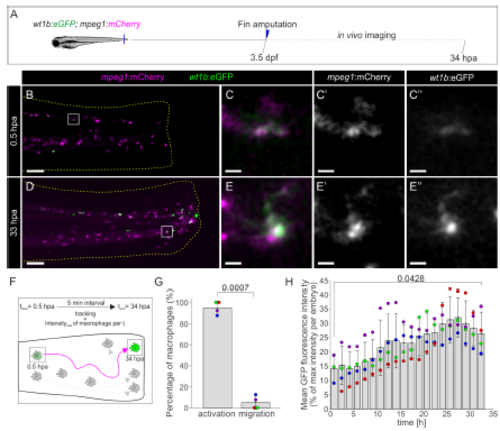
Activation of wt1b :eGFP during migration towards the amputation plane. Related to Figure 3. A, Caudal fins from Tg(wt1b:eGFP;mpeg1:mCherry) zebrafish larvae were amputated at 3.5 dpf and in vivo imaging was performed from 0.5 to 34 hpa. B, Overview of merged eGFP and mCherry channels at 0.5 hpa. C– C’’, Merged and single eGFP and mCherry channels of the zoomed views from boxed areas in panel B are shown. D, Overview of merged eGFP and mCherry channels at 33 hpa. E–E’’, Merged and single eGFP and mCherry channels of the zoomed views from boxed areas in panel B are shown. F, Scheme of macrophage tracking and fluorescence intensity measurement. wt1b:eGFP+ macrophages were tracked during the entire movie and the maximal GFP intensity within the macrophage was detected simultaneously. G, For each of 41 tracked macrophages in 4 embryos an intensity- and localization-based decision (25 % most anterior location and at least 33 % of the macrophage’s maximal intensity) defines whether they upregulate wt1b:eGFP during migration or are eGFP+ from the initial time of tracking. The graph shows the overall mean percentage of upregulation (activation) and migration as bars plus the percentage for each of 4 embryos. H, Overall mean GFP intensity of the 41 tracked macrophages at different time points (grey bars). Colored dots indicate mean GFP intensity Å} SD of macrophages in each of the 4 larvae. eGFP levels over time were normalized by maximal measured intensity per larvae. Timeintervals of 1.7 h (20 x 5 min) were binned. Paired t-test was performed. Scale bars: overview images 100 μm (B nd D), zoomed views 10 μm (C-C’’and E-E’’). |
|
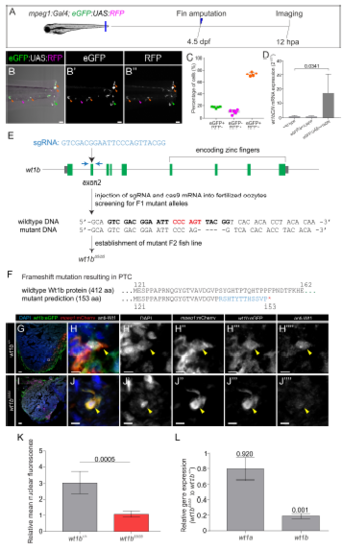
Validation of genetic lines to assess wt1b function. Related to Figures 3, 4 and 5. A-D, Validation of transgenic lines for macrophage-specific inhibition of wt1b function. A, Caudal fins of Tg(mpeg1:Gal4; GFP:UAS:RFP) larvae were amputated at 4.5 dpf and eGFP and RFP positive cells imaged at 12 hpa. B–B’’’, Images of the caudal fin showing RFP and eGFP positive cells. B’ and B’’ are single channels for eGFP and RFP. Orange arrowheads, double positive cells; magenta arrowheads, RFP+ cells; and green arrowheads, eGFP+ cells. C, Quantification of the percentage of eGFP+, RFP+ and double-positive cells. Dots indicate individual fish, shown are means Å} SD. Note that over 70% of macrophages are double-positive. D, Confirmation of wt1bDN overexpression using the Tg(eGFP:UAS:wt1bDN) line. Gal4FF mRNA was injected into 1-cell stage wildtype, embryos from the transgenic control line Tg(eGFP:UAS:RFP) or Tg(eGFP:UAS:wt1bDN). qRT-PCR was performed on cDNA obtained from these larvae. Shown are mean values Å} SD (n= 4 biological replicates per condition). Statistical analysis by Mann-Whitney test. E-L , Generation of the wt1bΔ5 mutant line. Related to Figures 4 and 5. E , Schematic drawing of the wt1b gene locus and workflow for wt1b mutant generation. A fish line that lacks 5 nucleotides in exon 2 of the wt1b gene (wt1bΔ5 ) was established. Blue arrows indicate the forward and reverse primer positions and the BsrI restriction site, used to detect indels, is highlighted in red. The sgRNA target sequence is shown in bold. F , The 5 nucleotides deletion is predicted to result in a frame shift (blue) and a premature termination codon that leads to a truncation of the protein. G-J’’’’ , Immunostaining with anti-WT1, anti-GFP and anti-mCherry on cryoinjured heart sections at 4 days postinjury from wt1b+/+ ; wt1b :eGFP;mpeg1 :mCherry and wt1bΔ5/Δ5 ; wt1b :eGFP;mpeg1 :mCherry zebrafish. Cell nuclei are counterstained with DAPI. Colocalization (arrowheads) of Wt1 with GFP and mCherry is detected in wt1b+/+ but absent in wt1bΔ5/Δ5 . K , Quantification of the relative mean Wt1 nuclear fluorescence in images of wt1b :eGFP; mpeg1 :mCherry cells normalized by background signal. Two-tailed unpaired t test. L , Quantitative RT-qPCR analysis of wt1a and wt1b expression in wt1bΔ5/Δ5 vs. wildtype larvae. Error bars represent standard error. p-values are calculated by pair wise fixed reallocation randomization with REST (n=10). dpf, days post fertilization; hpa, hours post amputation. Scale bars 50 μm (G and I), 40 μm (B-B’’) and 5 μm (H-H’’’’ and J-J’’’’). |
|
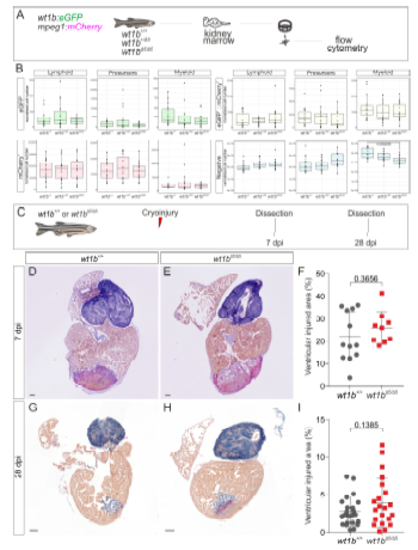
Phenotypic characterization of wt1bΔ5 mutants. Related to Figure 4 and Figure 5. Composition of the whole kidney marrow cell populations in wt1bΔ5 mutants. A, Whole kidney marrow (WKM) cells of wt1b+/+, heterozygous wt1b+/Δ5 or homozygous wt1bΔ5/Δ5 crossed into a Tg(wt1b:eGFP; mpeg1:mCherry) background fish were isolated. This is the same experiment as described in Figure 4, but here, eGFP+, mCherry+, double positive populations and the non-fluorescent fraction were analyzed by flow cytometry separately. B, Shown are boxplots of normalized cell numbers of cell populations in gate 1 (lymphoid), 2 (precursors) or 3 (myeloid). Normalized cell numbers relate to cell numbers per 106 events of living single cells. Negative (non-fluorescent) cell numbers in gate 3 are significantly lower in wt1bΔ5/Δ5 than in wt1b+/+ by one-way ANOVA followed by a Tukey’s post-hoc test. C-I, Fibrotic tissue deposition and regeneration in wt1bΔ5 mutants. C, Ventricular cryoinjury was performed to wt1b+/+ and wt1bΔ5/Δ5 adult fish and fibrosis assessed at 7 and 28 dpi on sectioned hearts using AFOG histological staining to detect collagen. D, E, Representative sagittal section of a wt1bΔ5/Δ5 and wt1b+/+ heart stained with AFOG at 7dpi. F, Quantification of injured area versus total ventricular area. Data from two independent experiments. Two-tailed unpaired t test. G-I, Representative images and quantification of injured cardiac ventricular area at 28 dpi, as shown in D-F. Two-tailed unpaired t test. dpi, days postinjury. Scale bars, 100 μm. |