- Title
-
Intravital Imaging Reveals Divergent Cytokine and Cellular Immune Responses to Candida albicans and Candida parapsilosis
- Authors
- Archambault, L.S., Trzilova, D., Gonia, S., Gale, C., Wheeler, R.T.
- Source
- Full text @ MBio
|
|
|
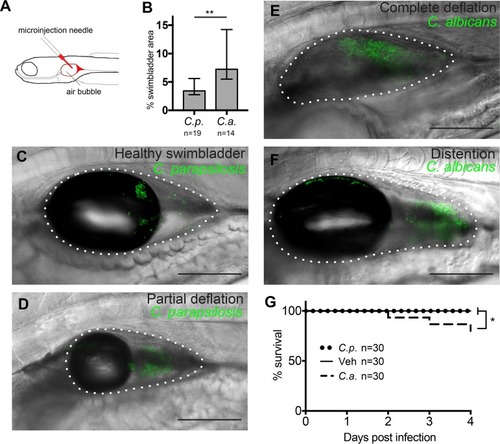
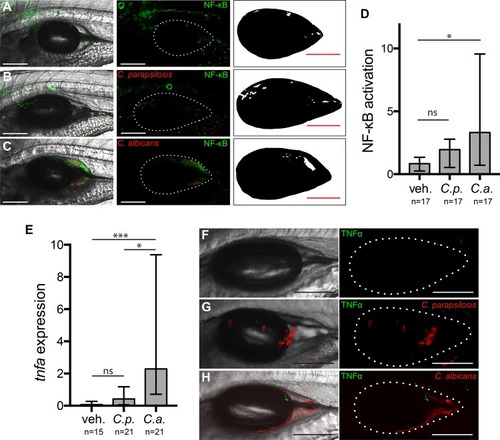
Transcription factor NF-κB is activated and proinflammatory cytokine TNF-α is expressed during |
|
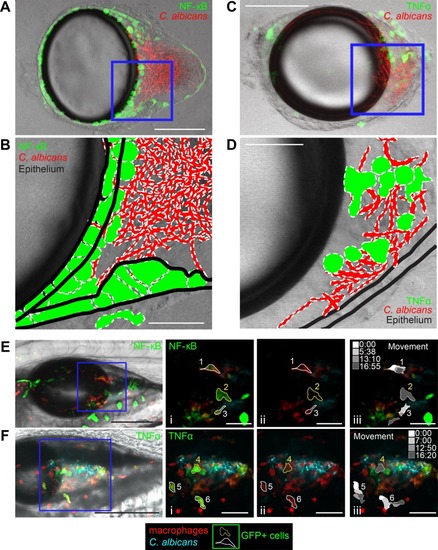
Patterns of NF-κB activation and TNF-α expression differ. Dissected swimbladders from |
|
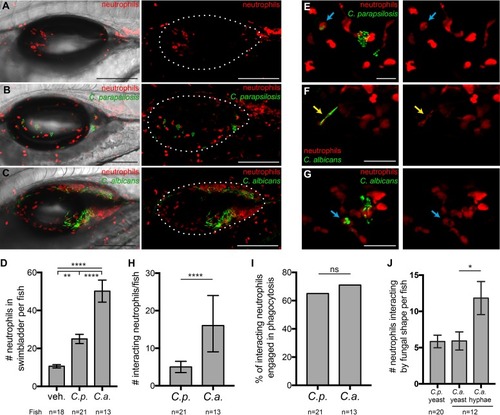
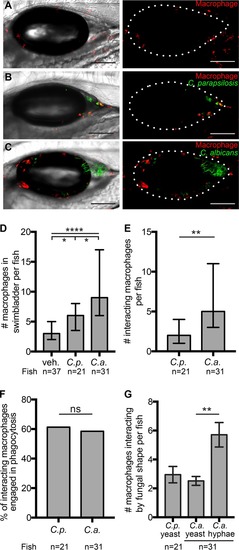
Neutrophils respond to infections with both |
|
Both |