- Title
-
Glycine Promotes the Survival of a Subpopulation of Neural Stem Cells
- Authors
- Bekri, A., Drapeau, P.
- Source
- Full text @ Front Cell Dev Biol
|
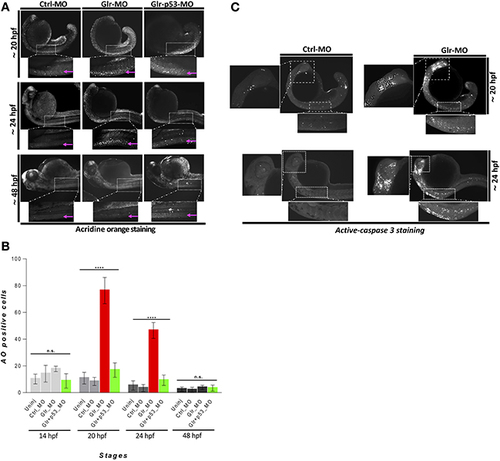
Disruption of glycine signaling causes an early and transient neural cell death during embryonic development. (A) Lateral views of zebrafish embryos stained by AO. live embryos injected by Ctrl-MO, Glr-MO, and p53-Glr-MO were stained by AO and imaged at 20, 24, and 48 hpf, higher magnification of cell death at spinal cord regions were highlighted in dotted boxed area. (B) Lateral view of zebrafish embryos at 20 and 24 hpf embryos injected by Glr-MO or Ctlr-MO and labeled by immunostaining for aCas3 (white dots), dotted box in the left and bottom showed a magnification of positives aCase3 in the brain and spinal cord. (C) Cell death quantification during zebrafish embryos development. AO positive cells at the spinal cord were quantified and compared in each condition, uninjected, Ctrl-MO, Glr-MO and p53-Glr-MO injected embryos at four-time points, 14, 20, 24, and 48 hpf. One-way ANOVA statistical analysis was performed (n = 15, ****p < 0.0001). EXPRESSION / LABELING:
PHENOTYPE:
|
|
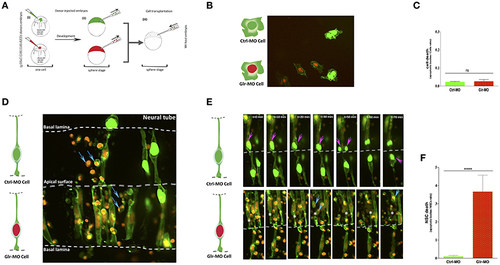
Defects of cell-autonomous glycine signaling induce a specific NSCs loss. (A) Cell transplantation strategy. Donor tg(HuC:Gal4,UAS:RFP) was injected at one-cell by mRNA to target membrane, cell nucleus and Glr-MO or Ctrl-MO as indicated in step (i). At the sphere stage, cells from both conditions were transplanted together in the same host wt embryo in the blastoderm margin (ii and iii). (B) Confocal z-series images of transplanted NSCs. Ctrl-MO transplanted cells showed a normal development to NSCs (NSCs with green membranes and nuclei). However, a considerable of apoptotic bodies were observed in Glr-MO transplanted cells (NSCs with green membranes and red nuclei). The basal and apical surface is outlined by a dotted line and double lines respectively. (C) Live imaging revealed programmed death of NSCs upon glycine signal disruption. Selected frames from 2 h time-lapse sequence of transplanted NSCs development showed a normal interkinetic nuclear migration of Ctrl-MO NSCs followed by cell division (top sequence, magenta arrows). In contrast, some of Glr-MO NSCs showed an arrest movement of interkinetic nuclear migration (bottom sequence, asterisk), followed by fragmentation of NSC to apoptotic bodies (bottom sequence, blue arrows). (D) Quantification of transplanted NSCs death (apoptotic bodies / NSC ratio) in both conditions, Glr-MO (and Ctrl-MO reveal a drastic NSCs death upon disruption of glycine signaling by more than folds. (E) Disruption of glycine signaling does not affect non-neuronal cells survival. Transplanted Glr-MO cells (red nuclei) and Ctrl-MO cells (Green nuclei) were developed naturally to non-neural cells (skin) without showing apoptotic bodies formation. (F) Quantification of transplanted non-neural cell in both conditions, Glr-MO and Ctrl-MO reveal no significant cell death upon disruption of glycine signaling. t-test statistical analysis was performed (n = 6, ****p < 0.0001). |
|
Glycine signaling defect does not affect GFAP+ NSCs subpopulation. (A) Using GFAP as a marker of NSCs subpopulation, GFAP transcripts were analyzed by whole-mount in situ hybridization. No major difference of GFAP+ subpopulation was observed between Ctrl-MO (i), and Glr-MO (ii) conditions at 15 and 24 hpf. (B) Double tg(GFAP:GFP; HuC:RFP) line was used to analysis GFAP expression (white) and HuC+ expression as mature neurons marker (Bleu). GFAP+ signal does not change between Ctrl-MO (i) and Glr-MO (ii) conditions at 24 and 48 hpf. However, a clear decreasing of HuC+ signal was observed between Ctrl-MO (i), and Glr-MO (ii) conditions. (C) Quantification of GFAP-GFP embryos upon disruption of glycine signaling does not affect GFAP+ subpopulation. For both ISH and IHC, n > 20 embryos per sample. IHC, immunohistochemistry; ISH, in situ hybridization. EXPRESSION / LABELING:
|
|
Glycine signaling defects induces a drastic loss of nestin+ NSCs subpopulation. (A) Using nestin as a marker of NSCs subpopulation, nestin transcripts were analyzed by whole-mount in situ hybridization. A drastic loss of nestin+ subpopulation was observed between Ctrl-MO (i), and Glr-MO (ii) conditions at 15 and 24 hpf. (B) Double tg(GFAP:GFP; HuC:RFP) line was used to analysis nestin (white) and HuC+ (Bleu) expressions. Major reduction of nestin+ signals in Glr-MO conditions (ii) compared with Ctrl-MO (i) at 24 and 48 hpf. In addition to reduction of HuC+ signals in Glr-MO condition (ii). (C) Quantification of nestin-GFP embryos upon disruption of glycine signaling reveal a drastic reduction of GFP signal compared with control. However, co-injecting of p53-MO and Glr-MO rescue partially nestin+ subpopulation. For both ISH and IHC, n > 20 embryos per sample. IHC, immunohistochemistry; ISH, in situ hybridization. EXPRESSION / LABELING:
PHENOTYPE:
|