- Title
-
Role of Calcium-Sensing Receptor in Mechanotransducer-Channel-Mediated Ca2+ Influx in Hair Cells of Zebrafish Larvae.
- Authors
- Lin, L.Y., Yeh, Y.H., Hung, G.Y., Lin, C.H., Hwang, P.P., Horng, J.L.
- Source
- Full text @ Front. Physiol.
|
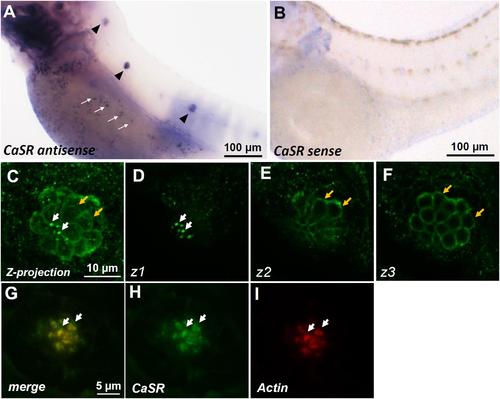
In situ hybridization and immunocytochemistry of the Ca2+-sensing receptor (CaSR) in zebrafish larvae. CaSR mRNA was expressed in neuromasts (arrowheads) and ionocytes (arrows) of 3- day-postfertilization (dpf) larvae (A). The sense probe detected no signal in larval skin (B). Confocal images of CaSR antibody-labeled lateral-line neuromasts of 4-dpf larvae (C, a Z-projection). Confocal z-stacks of the same neuromast revealed that the CaSR was expressed in stereocilia (white arrows in C,D) and basolateral membranes (orange arrows in C,E,F). Double immunocytochemical labeling of the CaSR (H) and actin (I) in 4-dpf larvae; the merged image is shown in (G). White arrows indicate the colocalization of both CaSR and actin signals in stereocilia. EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
Ca2+-sensing receptor (CaSR) protein expression in neuromast hair cell of CaSR morpholino oligonucleotide (MO) knockdown larvae. Immunocytochemical staining with CaSR antibodies was conducted in 4-dpf wild-type larvae (A,B), control MO-injected larvae (control-MO; C,D), and CaSR-MO-injected larvae (CaSR-MO; E–H). CaSR signals (green) were detected in the apical (arrows; A,C) and basolateral (arrows; B,D) membranes of hair cells in wild-type and control-MO larvae. In CaSR-MOs, the CaSR signal was weak in the apical and basolateral membranes (E–H). Scanning electron microscopy images revealed the morphologies of L1 neuromat hair bundles in 4-dpf control-MOs and CaSR-MOs (arrowheads; I,K,M). Immunocytochemical staining with S100 antibodies was conducted in 4-dpf control-MOs and CaSR-MOs (J,L,N). The hair cell numbers of L1 neuromasts in the posterior lateral lines were determined (O). Data are presented as mean ± SE. No significant differences were identified (one-way ANOVA and Tukey’s comparison, p < 0.05). Numbers in parentheses are the numbers of neuromasts. One L1 neuromast per larva was examined. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
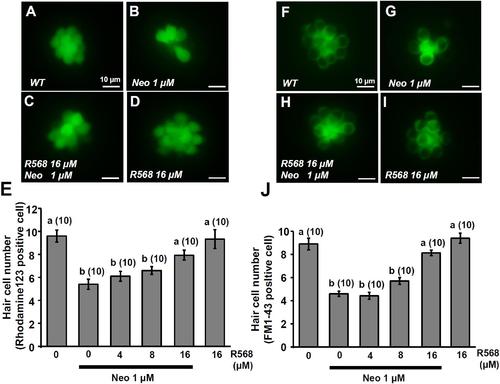
Effect of R-568 on 1 μM neomycin-induced hair cell death. 4-dpf larvae were incubated in 1 μM neomycin media with various levels of R-568 (E,J) for 1 h. After treatment, neuromast hair cells were labeled with rhodamine 123 (A–E) or FM1-43 (F–J). R-568 at 16 μM neutralized the neuromast hair cell death caused by 1 μM neomycin (E,J). Data are presented as mean ± SE. A significant difference was found between a and b (by one-way ANOVA and Tukey’s comparison, p < 0.05). Numbers in parentheses are the numbers of neuromasts. One L1 neuromast per larva was examined. PHENOTYPE:
|
|
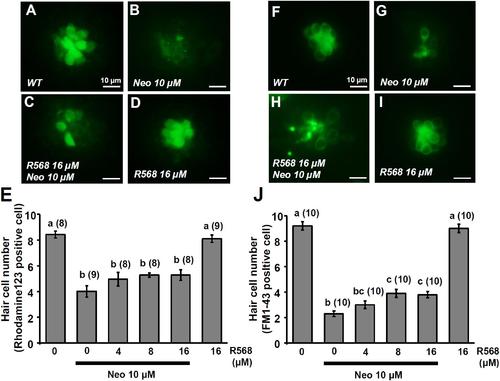
Effect of R-568 on 10 μM neomycin-induced hair cell death. 4-dpf larvae were incubated in 10 μM neomycin media with various levels of R-568 (E,J) for 1 h. After incubation, neuromast hair cells were labeled with rhodamine 123 (A–E) or FM1-43 (F–J). The addition of 16 μM R-568 partial neutralize the neuromast hair cell death induced by 10 μM neomycin (J). Data are presented as mean ± SE. A significant difference was found between values with superscript letters (a,b,c; by one-way ANOVA and Tukey’s comparison, p < 0.05). Numbers in parentheses are the numbers of neuromasts. One L1 neuromast per larva was examined. PHENOTYPE:
|