- Title
-
Optical mapping of neuronal activity during seizures in zebrafish
- Authors
- Turrini, L., Fornetto, C., Marchetto, G., Müllenbroich, M.C., Tiso, N., Vettori, A., Resta, F., Masi, A., Mannaioni, G., Pavone, F.S., Vanzi, F.
- Source
- Full text @ Sci. Rep.
|
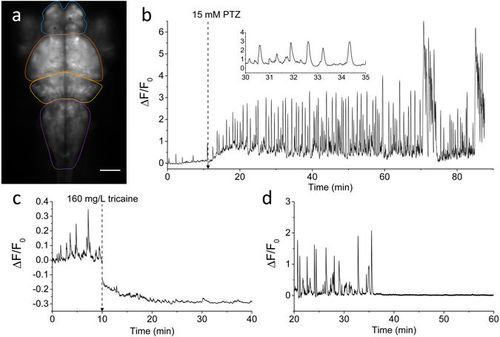
Brain activity recording with GCaMP6s. (a) Fluorescence image of the encephalon of a 4 dpf larva expressing GCaMP6s under elavl3 promoter. Scale bar: 100 µm. The four main zebrafish brain regions used for further analysis in the paper are highlighted (Telencephalon, blue; Optic tectum, orange; Cerebellum, yellow; Medulla, purple). (b) Time trace of fluorescence (ΔF/F0, see Methods for detail) in control conditions and after addition of 15 mM PTZ (at the time indicated). The fluorescence data are integrated over the whole brain of the larva and sampled at 5 Hz. The inset shows a shorter time interval of the trace to better display calcium peak shape and regularity. (c) ΔF/F0 trace of an experiment in which tricaine (160 mg/L) was added at the time indicated and the subsequent decay of activity was monitored. The ΔF/F0 values become negative after tricaine addition since the F0 reference level was measured in control conditions (see Methods for details). (d) ΔF/F0 trace showing sharp brain activity decay in a larva simultaneously treated with 15 mM PTZ and 5 μM TTX. After approximately 35 min upon drugs application, TTX action totally suppressed neuronal seizure activity induced by PTZ. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
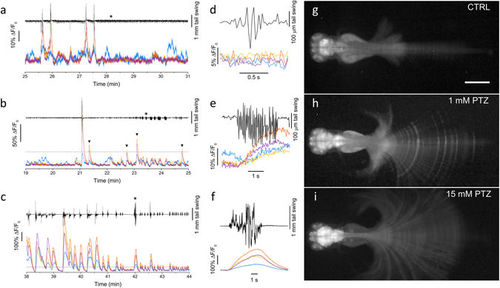
Simultaneous measurement of brain activity and tail movement. (a) Time trace of tail movement (black line) and fluorescence (ΔF/F0) analysed in control conditions (0 mM PTZ) for four brain regions color-coded as in Fig. 1a. The traces show correlations between fluorescence peaks and tail movements for optic tectum, cerebellum and medulla, while the telencephalon exhibits some degree of activity not correlated with tail movement. (b-c) Tail movement and brain activity analysed as in panel a at 1 and 15 mM PTZ, respectively. The dashed lines show a ΔF/F0 level of 0.4 corresponding to the highest peaks typically measured in control conditions. The arrowheads in panel b highlight peaks overcoming the 0.4 level and not corresponding to large tail movements. (d-e-f) Expanded time scale traces of the fragment indicated by the asterisk in a, b and c, respectively. Each panel shows movement and fluorescence traces (color-coded as in panels a-c) corresponding to a typical tail movement observed in the corresponding condition. (g-h-i) Fluorescence images of head-restrained larvae (see Methods) in 0, 1 and 15 mM PTZ. Images are maximum intensity projections over a time interval of 10 min, chosen during typical locomotor activity of the tested condition. Panels h and i are displayed with gamma = 0.4 to avoid saturation in the encephalon and still have good contrast on the tail. Scale bar 500 µm. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
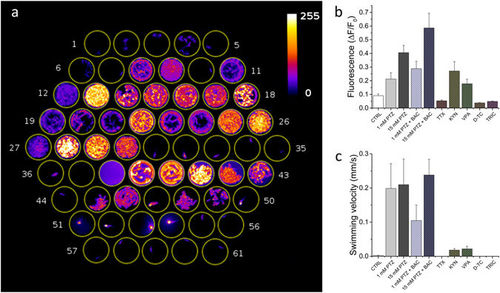
High-throughput combined fluorescence and behavioural assay. (a) Maximum intensity projection of a 13 minute segment of the recording. Well content: 1–6 control, 7–12 1 mM PTZ, 13–18 15 mM PTZ, 19–24 1 mM PTZ + 50 μM Baclofen, 25–30 15 mM PTZ + 50 μM Baclofen, 31–36 15 mM PTZ + 5 μM Tetrodotoxin, 37 fish water, 38 fish water + 2 mM Kynurenate, 39–43 15 mM PTZ + 2 mM kynurenate, 44–49 15 mM PTZ + 5 mM Valproate, 50–55 15 mM PTZ (after pre-incubation in 2 mM d-tubocurarine for 10 minutes before starting the measurement), 56–61 15 mM PTZ + 160 mg/L Tricaine. (b) Average ΔF/F0 and (c) swimming velocity for the different conditions tested. Error bars: s.e.m. |
|
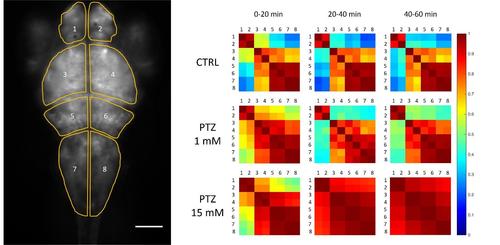
Cross-correlation maps of activity in different brain regions. Left. Regions of interest selected for analysis are shown overlaid with the fluorescence image; scale bar 100 μm. Right. Cross-correlation matrices (see Methods) measured at different time intervals during a one-hour recording in different conditions, as indicated. Each matrix shows color-coded mean correlation coefficients of three larvae, exposed to the same condition, during the same timeframe. |
|
Evaluation of cross-talk between brain regions due to wide-field imaging. Imaging was performed on the same larva in wide field (left, top panel) and confocal (left, middle panel). The confocal image was constructed as the sum of 183 confocal planes (with 2 μm z-steps) encompassing the whole depth of the larva brain. This reconstruction is taken as a good reference for comparison with the wide field image, since each plane does not suffer from contributions of out-of-focus planes from adjacent brain regions at different depths. Comparison of the two images and observation of the overlay (left, bottom panel) demonstrates that the wide field image captures most of the relevant features of the encephalon as imaged with confocal microscope. A quantitative correlation between wide-field versus confocal intensities of the four regions analysed in the paper is shown in the right panel. Intensities are reported as percentage over the total intensity integrated on the whole brain. |