- Title
-
Arl13b Interacts With Vangl2 to Regulate Cilia and Photoreceptor Outer Segment Length in Zebrafish
- Authors
- Song, P., Dudinsky, L., Fogerty, J., Gaivin, R., Perkins, B.D.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
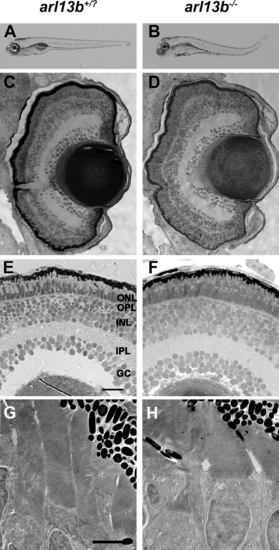
arl13b is not required for zebrafish retinal development. (A, B) Lateral view of larvae at 5 dpf. (C-F) Methylene blue-stained plastic sections of 5 dpf wild-type siblings (arl13b+/?) and arl13b-/- mutant retinas. The outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), and ganglion cell layer (GC) were present in wild-type and mutant retinas. (G, H) Transmission electron micrographs of 5 dpf retinas do not show any evidence of photoreceptor outer segment disorganization or ciliary defects. Scale bars: 200 µm (A, B), 20 µm (C, D), 10 µm (E, F), and 2 µm (G, H). PHENOTYPE:
|
|
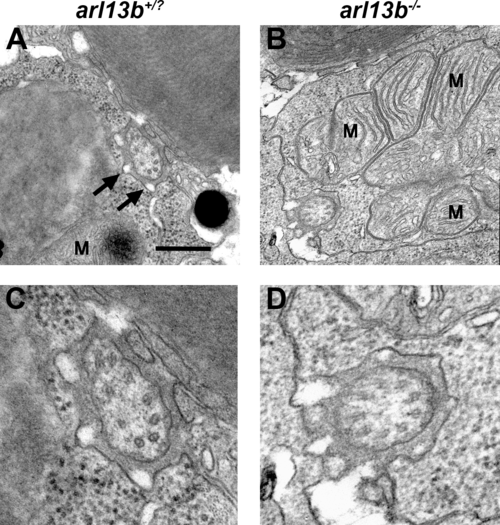
Ultrastructural analysis of photoreceptor connecting cilia by transmission electron microscopy. (A, B). Horizontal sections through photoreceptors of wild-type and arl13b-/- larvae at 5 dpf show the connecting cilia in the vicinity of the mitochondria (M). Calyceal processes surround the cilium (arrows). (C, D) Higher magnification images revealed no obvious defects in microtubule organization in arl13b-/- mutants. Scale bars: 0.5 µm (A, B) and 0.2 µm (C, D). PHENOTYPE:
|
|
arl13b-/- mutants have shorter photoreceptor outer segments and cilia at 4 dpf. (A, B) Immunohistochemistry on transverse cryosections from 4 dpf wild-type and arl13b-/- mutant retinas stained for rhodopsin (red). (C) Quantification of the length of rhodopsin staining along the long axis of the photoreceptor. (D, E) Immunohistochemistry for acetylated α-tubulin (red) and Ift88 (green). (F) Quantification of average cilia length. All sections were counterstained with DAPI to label nuclei. Scale bars: 10 µm. Error bars: ± SEM. *P < 0.0001 using Student′s t-test with Welch′s correction. |
|
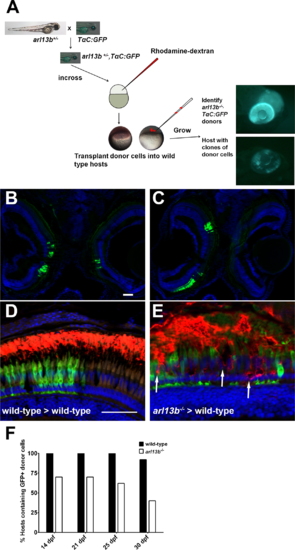
Progressive degeneration of arl13b-/- photoreceptors. (A) Overview of the blastula transplant strategy. Heterozygous arl13+/- fish were crossed to the TαC:GFPucd1 transgenic line to produce arl13b+/-; TαC:GFPucd1 animals. Progeny from heterozygous crosses were injected with rhodamine-dextran and donor cells transplanted into the animal pole of unlabeled wild-type hosts. At 4 dpf, arl13b-/-;TαC:GFPucd1 mutants were identified phenotypically (top). Wild-type hosts containing photoreceptors from mutant donors were identified by mosaic GFP fluorescence within the eye (bottom). (B, C) Cryosections of 14 dpf larvae showing GFP+ clones populating retinas bilaterally and unilaterally. (D, E) Immunofluorescence image of 16 dpf larval retinas immunolabeled for rhodopsin (red). Cone photoreceptors from donor embryos express GFP (green). Transplanted cone photoreceptors from an arl13b-/-; TαC:GFPucd1 mutant donors exhibited partial rhodopsin mislocalization within the donor clone ([E], arrows). Sections were counterstained with DAPI to show nuclei. (E) Graph showing the percentage of hosts (n > 11 retinas per genotype) still containing GFP-positive cells from wild-type or arl13b-/- mutant donors at specified time points. Scale bar: 50 µm. |
|
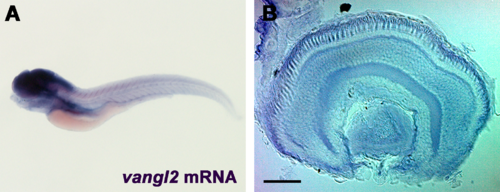
Expression of vangl2 in 5 dpf zebrafish larvae. (A) Whole-mount in situ hybridization of vangl2 in 5 dpf larvae shows expression in the central nervous system. (B) Retinal expression was highest in the RPE and photoreceptor layers. Scale bar: 40 µm. EXPRESSION / LABELING:
|
|
Suppression of arl13b and PCP components result in shortened photoreceptor cilia and outer segments. (A-D) Immunohistochemistry using antibodies against acetylated α-tubulin (red) to label cilia in retinal cryosections of 5 dpf zebrafish larvae. (E-H) Immunohistochemistry using rhodopsin antibodies to label photoreceptor outer segments in retinal cryosections of 5 dpf larvae. (I) Quantification of cilia length. (J) Quantification of outer segment area. All sections were counterstained with DAPI. Results are significant (****P < 0.0001) using a 1-way ANOVA with Tukey′s multiple comparisons test correction. Error bars: ± SEM. Scale bar: 10 µm. |
|
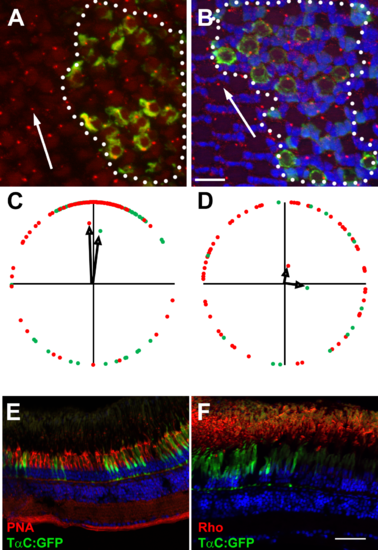
Vangl2 is not essential for photoreceptor outer segments. (A, B) Representative fields of cells showing basal bodies of (A) red-/green-sensitive cones and (B) UV-sensitive cones. Basal bodies were stained by γ-tubulin (red) and GFP (green) marked vangl2-/-;Tg(-3.2gnat2:GFP) donor cells. Clones of donor cells are marked with white dotted lines. Nuclei were counterstained with DAPI (blue). (C, D) Angular dimensions of wild-type (red points) and vangl2-/- mutant basal bodies (green points) were plotted around a unit circle. The mean vectors are indicated as black arrows. The optic nerve is up in all Figures. (E, F) Transverse cryosections of mosaic animals containing vangl2-/-;Tg(-3.2gnat2:GFP) donor cells and stained for PNA or rhodopsin (red). No differences were seen in cells inside the clone versus those outside the clone. Scale bars: 10 µm (A, B) or 50 µm (E, F). |