- Title
-
Essential roles of zebrafish rtn4/Nogo paralogues in embryonic development
- Authors
- Pinzón-Olejua, A., Welte, C., Abdesselem, H., Málaga-Trillo, E., Stuermer, C.A.
- Source
- Full text @ Neural Dev.
|
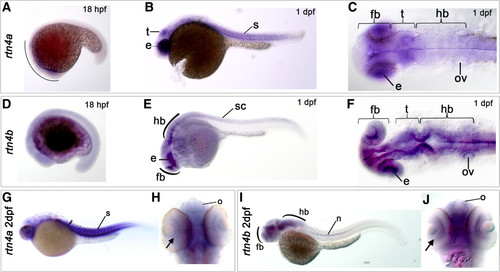
Expression of rtn4a and rtn4b genes during zebrafish embryogenesis. The developmental expression pattern of rtn4a and rtn4b was examined by whole-mount in situ hybridization using gene-specific probes. (A) At 18 hours postfertilization (hpf), rtn4a is expressed in the anterior part of the embryo. Between 1 day postfertilization (dpf) (B) and (C) and 2 dpf (G) and (H), we detected increased transcription of rtn4a in the somites (s) and the eye anlage (e), as well as in the presumptive optic tectum (t). (D) At 18 hpf, rtn4b transcripts appeared along the trunk of the embryo. (E) and (F) At 1 dpf, rtn4b expression is evident in the posterior spinal cord (sc), the developing forebrain (fb), eye anlagen (e) and midbrain and hindbrain (hb), including the otic vesicle (ov). (G) and (H) At 2 dpf, rtn4a mRNAs are produced in retinal ganglion cells (RGCs) (arrow), the olfactory organ (o) and forebrain (fb), as well as in somites (s). (I) and (J) At 2 dpf, rtn4b is transcribed in the forebrain (fb), including the olfactory organ (o) and RGCs (arrow), and in the midbrain and hindbrain. At this stage, the spinal cord was no longer labeled, but the notochord (n) began to express rtn4b. Lateral views are shown, except in (C) and (F) (dorsal views of 1-dpf embryos) and (H) and (J) (ventral views of 2-dpf embryos). EXPRESSION / LABELING:
|
|
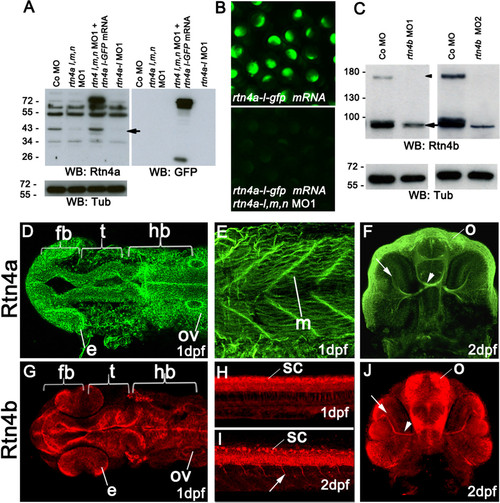
Rtn4a and Rtn4b protein levels and morpholino knockdown. (A) Western blot (WB) analysis using the zebrafish α-Rtn4a antibody 15] shows that Rtn4a-l (an approximately 43-kDa band, arrow) is suppressed either by injection of a mixture of morpholinos (MOs) against each rtn4a isoform or a MO against the rtn4a-l isoform. Embryos expressing an Rtn4a-l-GFP fusion can overcome the Rtn4a MO1 downregulation. GFP antibodies detect the fusion protein at approximately 70 kDa and GFP alone at approximately 26 kDa. α-tubulin (Tub) served as a protein loading control. (B) Exogenous Rtn4al-GFP was detected at 6 hpf, but simultaneous coinjection of rtn4a-l MO1 abrogated its expression. (C) α-Rtn4b antibodies show the downregulation of Rtn4b for both MOs used in the experiments. The 180-kDa band (arrowhead), probably a dimerization band, is entirely reduced in MO-injected embryos, and the 90-kDa band (arrow) shows a strong reduction. (D) through (J) Rtn4a and Rtn4b immunostainings at various developmental stages. At 1dpf, Rtn4a is expressed in distinct neuronal structures, including the forebrain (fb), the presumptive optic tectum (t) and the hindbrain (hb). Rtn4a is also present in the eye anlage (e), otic vesicle (ov) (D), and in muscle tissue (m) (E). At 2 dpf, Rtn4a is detected in retinal ganglion cells (RGCs) (arrow), the optic nerve (arrowhead) and the olfactory system (o) (F). At 1 dpf, Rtn4b is expressed in the same structures as Rtn4a except the muscle tissue (G). Rtn4b is also detected in the spinal cord (sc) (H). At 2 dpf, the Rtn4b signal is still present in the spinal cord (sc) and can also be seen in growing primary motor neurons (arrow) (I). In the head, Rtn4b is present in RGCs (arrow) and the optic nerve (arrowhead) (J). |
|
Morphogenetic defects after morpholino-mediated downregulation of rtn4a and rtn4b. (A) At 15 hpf, control embryos showed a differentiated eye anlage (e) and forebrain (arrow). In contrast, the general morphology of rtn4a- and rtn4b-morpholino (MO)-injected embryos was visibly affected (D) to (G). The forebrain was flattened (arrow), and the eye anlage (e) and the head were reduced in size. At 1 dpf, the heads and eyes of embryos injected with rtn4a and rtn4b MO1 remained reduced. In particular, rtn4b morphants exhibited an abnormally curved notochord (E) to (H). At 2 dpf, the rtn4a morphants still had reduced eyes and forebrains compared to controls (Co) (F and F2 vs. C and C2), but no other morphogenetic defects were apparent. Rtn4b morphants had even smaller eyes, markedly shortened forebrain/midbrain regions and a deformed fourth ventricle (I and I2, arrow). At 3 dpf, the eyes and brains of rtn4a and rtn4b morphants remained smaller (J, L and N). Rtn4b morphants developed a thinner, ventrally curved tail; lacked lower jaws (asterisk); and had an inflated heart cavity. At 5 dpf, rtn4a, but not rtn4b, morphants seemed to regain a nearly normal overall morphology (K, M and O). The reduction in eye size was quantified at 15 hpf in embryos injected with rtn4b MO1 and MO2 (targeting the 52 untranslated region and ATG, respectively) and coinjected with rtn4b mRNA (P). In rescue experiments, the rtn4b morphant phenotype showed clear improvement (Q). Anterior brain structures selected for measurements are outlined in yellow. Samples studied were rtn4b MO1 (n = 36), rtn4b MO2 (n = 64), rtn4b MO1 + rtn4b mRNA (n = 69) and rtn4b MO1 (n = 85). Scale bars = 100 μm. PHENOTYPE:
|
|
Development of neural and non-neuronal structures in rtn4b-knockdown, GFP transgenic embryos. At 1 dpf, tg(Shh:GFP), rtn4b morphant embryos showed deformations in the hypothalamic region (h), dorsal thalamic structures (arrow), anterior floor plate (f) and forebrain (A) and (B). Additionally, the notochord (n) is abnormally undulated (compare (C) and (D)). In addition, 1-dpf tg(hb9:GFP), rtn4b morphant embryos had fewer cell bodies in the spinal cord and truncated motor axons (E) and (F) (arrowheads). At 2 dpf, motor axons showed aberrant branching (G) and (H) (arrows). At 5 dpf, lateral (J) and dorsal (L) views reveal smaller eyes (e), aberrant retinal ganglion cells (RGCs) (white arrow) and few retinal axons projecting into the tectum (t), which is displaced anteriorly, along with abnormally patterned branchial arches (br) and reduced pectoral fins (pf) (I) to (L). At 2 dpf, tg(Isl1:gfp), rtn4b-MO1 morphants showed severe defects in cranial motor neurons, including lack of pairs III and IV (M) and (N). (O) Quantification of the number of motor neurons in posterior segments (n = 15 to 20) of the spinal cord in control morpholino (Co MO; n = 20), rtn4b-MO1 (n = 25) and rescued (n = 20) embryos at 1 dpf. (P) Proportion of embryos with abnormal motor axons in embryos injected with Co MO, rtn4b-MO, rtn4b-MO2, or coinjected with half concentrations of both MOs, or rtn4b-MO1 + rtn4b-mRNA, and injected with rtn4b-mRNA. hb: hindbrain; e: eye; ob: olfactory bulb; c: cerebellum; X: branchiomeric nerve X. Co MO (n = 15), rtn4b-MO1 (2.5 ng) (n = 30), rtn4b-MO2 (2.5 ng) (n = 31), rtn4b-MO1 (5.0 ng) (n = 17), rtn4b-MO2 (5.0 ng) (n = 11), rtn4b-MO1 + rtn4b-MO2 (2.5 ng) (n = 20), rtn4b-MO1 (5.0 ng) + rtn4b-mRNA (100 pg) (n = 69) and rtn4b mRNA (100 pg) (n = 85). Scale bar = 100 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
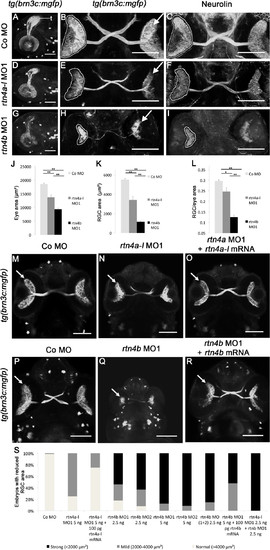
Retinal ganglion cell development in rtn4a- and rtn4b-knockdown, GFP–transgenic embryos. (A) through (I)tg(brn3c:mGFP) embryos at 3 dpf exhibit labeled retinal ganglion cells (RGCs) in the eye (arrow) and RGC axons in the optic nerve and tract as well as in the optic tectum (t). (A), (D) and (G) Lateral views of the left eye and optic tectum (anterior to the left). (B), (C), (E), (F), (H) and (I) Ventral views (anterior to the top). (A) to (C) In control morpholino (Co MO)–injected embryos, RGC axons reached the optic tectum (t) and innervated the neuropil (A). The ventral view shows the RGCs in both eyes (arrow) and the RGC axons in the optic nerve, the chiasm and the optic tract (B). (C), (F) and (I) RGCs and their axons were costained with antibodies against neurolin. RGCs from both morphants, rtn4a(D) to (F) and, to a greater extent, rtn4b(G) to (I), cover smaller areas of the retina (outlined) and their axons show aberrant pathways. (J) to (L) Quantification of eye size, the area covered by RGCs and their ratio. (M) to (O) and (P) to (R) Rescue of the rtn4a and rtn4b RGC phenotypes. Arrow points to RGCs. (S) Quantification at 3 dpf showing the reduction in the area covered by RGCs after injection of rtn4a- and rtn4b-MO1 (separately and combined) and rtn4b-MO1 and rtn4b-mRNA, as indicated under each bar. Control-MO (n = 28), rtn4a-l-MO1 (5.0 ng) (n = 16), rtn4a-l-MO1 (2.5 ng) + rtn4a-l-mRNA (100 pg) (n = 32), rtn4b-MO1 (2.5 ng) (n = 22), rtn4b-MO2 (2.5 ng) (n = 24), rtn4b-MO1 (5.0 ng) (n = 19), rtn4b-MO2 (5.0 ng) (n = 24), rtn4b-MO1 and rtn4b-MO2 (2.5 ng) (n = 27), rtn4b-MO (5.0 ng) and rtn4b-mRNA (100 pg) (n = 23) and rtn4a-l-MO1 (2.5 ng) and rtn4b-MO1 (2.5 ng) (n = 27). Scale bar = 100 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Brain differentiation and retinotectal projections in rtn4a- and rtn4b-knockdown, GFP–transgenic embryos. In rtn4a-morpholino (MO)- and rtn4b-MO-injected embryos, brains are smaller both anteroposteriorly as well as laterally. (A) Extent of the tectum (t) and the forebrain (fb) marked by white brackets in the HuC/HuD labeled brains of control embryos. (D) and (G) Knockdown of rtn4a, but more severely of rtn4b, leads to a reduction in the size of the tectum and the forebrain. In rtn4b morphants, the olfactory placodes (arrowhead) are not clearly identifiable and the tectum is localized in abnormally anterior positions. (B) In control embryos, the GFP–labeled retinal ganglion cell (RGC) axons cover the tectal neuropil (outlined). (E) and (H) The RGC axons in the tectum cover a smaller area in both morphants, but more severely so in the rtn4b morphants than in their rtn4a counterparts. GFP is also expressed in neuromasts (arrows), which are aberrantly positioned in the morphants. (C), (F) and (I) 42,6-diamidino-2-phenylindole (DAPI) staining of the brain shows the area of the neuropil (outlined), which is reduced after downregulation of rtn4a and rtn4b. (J), (K) and (L) Quantification of the length of the optic tectum, forebrain and tectal neuropil in control and rtn4a-MO- and rtn4b-MO-injected embryos. (A) to (I) Dorsal views of the brain of tg(brn3c:mGFP) embryos at 5 dpf. Control MO (5.0 ng) (n = 10), rtn4a-l-MO1 (5.0 ng) (n = 10) and rtn4b-MO1 (5.0 ng) (n = 10). Scale bar = 100 μm. |
|
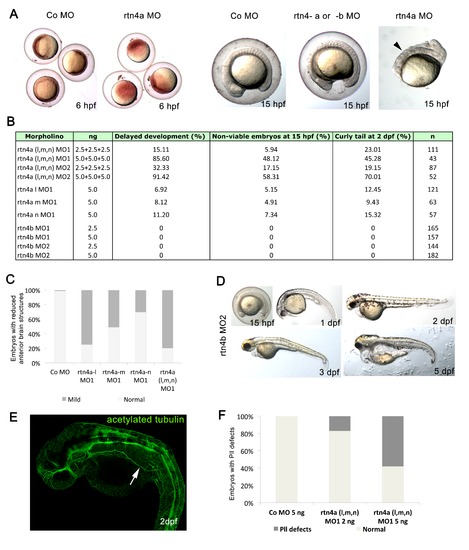
Toxicity of rtn4a morpholino-mediated knockdown. (A) Impaired development and viability after injection of rtn4-morpholino (MO). Embryos were microinjected at cell stages 1 to 4 and analyzed at 6 hpf and 15 hpf. Higher doses of rtn4a-MO resulted in delayed gastrulation at 6 hpf and reduced viability at 15 hpf compared to control embryos (arrowhead). (B)rtn4a and rtn4b MOs were microinjected at 2.5 or 5.0 ng. Mortality, development and viability were evaluated for all experimental groups in at least three experiments. Embryos showing a curly tail phenotype upon Rtn4a knockdown were excluded from the experimental group. (C) Quantification of embryos with reduced anterior brain structures was carried out after knockdown of each Rtn4a isoform. Control (n = 95), rtn4a-l MO1 (n = 78), rtn4a-m MO1 (n = 23), rtn4a-n MO1 (n = 33) and rtn4a (l, m and n) MO1 (n = 78). (D) A second rtn4b-MO sequence (rtn4b-MO2) elicited the same phenotype as the rtn4b-MO1 sequence. At 15 hpf, the forebrain was flattened and the sizes of the head and eye anlage were reduced. At 1 dpf, rtn4b-MO2-injected embryos were smaller than control embryos, with reduced heads and eyes. Embryos also had an abnormally curved notochord. At 2 dpf, rtn4b morphants had shortened fore- and midbrain regions. At 3 dpf, rtn4b morphants developed a thinner tail, which curved ventrally, lacked lower jaws and had an inflated heart cavity. At 5 dpf, they did not recover any of the abnormalities seen at 3 dpf. (E) Acetylated tubulin staining of 2-dpf embryos injected with 5 ng of rtn4a MO revealed pathfinding mistakes of the lateral line. (F) Proportion of embryos with aberrant pathfinding of the lateral line at 2 dpf after injection of a mixture of 2 ng (n = 45) or 5 ng (n = 75) of each isoform of rtn4a-MO (arrow). |
|
Immunostaining of morphant embryos confirms the specificity of Rtn4a and Rtn4b antibodies. (A) In 1 dpf control morpholino-injected embryos, the Rtn4a antibody labeled neural structures such as the neural tube. Upon morpholino knockdown of all rtn4a isoforms (C) or the rtn4a-l isoform only (E), the signal appeared clearly reduced. Similarly, at 2 dpf, labeling of retinal ganglion cells (RGCs) and optic nerves (arrow) in control embryos (B) was reduced after knockdown of all or only the rtn4a-l isoforms (D) and (F). (G) and (H) In control embryos, antibodies against Rtn4b labeled RGCs (arrow) (G), spinal cord (arrow) and motor neurons (arrowheads) (H). The signal in these structures was drastically reduced after Rtn4b downregulation (I) and (J). (A), (C) and (F) show dorsal views (rostral to the left). (B), (D), (F), (G) and (I) show ventral views (rostral at the top). (H) and (J) show lateral views (rostral to the left). |
|
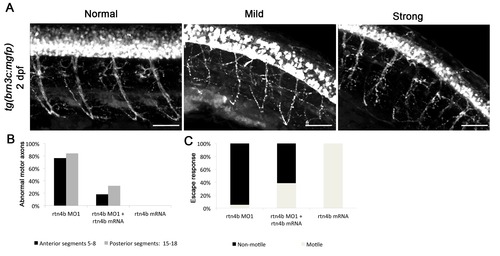
Rtn4b morphants in brn3c:mGFP transgenic embryos. (A) Branching of motor neurons in rtn4b morphants. Axonal projections (trunk segments 5 to 8 and 15 to 18) were analyzed. In mild phenotypes, motor axons showed misbranching and pathfinding mistakes, whereas in strong phenotypes defasciculation was also observed. (B) Proportion of abnormal motor axons in anterior and posterior segments in rtn4b morphants and in the rescue group at 2 dpf. rtn4b MO1 (n = 19), rtn4b-MO1 and rtn4b-mRNA (n = 25) and rtn4b-mRNA (n = 20). (C) Proportion of nonmotile embryos at 3 dpf in rtn4b morphants, rescued and rtn4b-mRNA-injected groups. |
|
Apoptosis in rtn4 morphant embryos. Comparison of apoptosis in control (A), rtn4a morphant (B) and rtn4b morphant (C). Cell death was visualized at 1 dpf by acridine orange staining. (D) and (E) Quantification of acridine orange intensity in selected areas of the midbrain (square) and hindbrain (rectangle) showing increased staining (arrow) in both morphants. Control MO (5.0 ng) (n = 30), rtn4a-l-MO1 (5.0 ng) (n = 25) and rtn4b-MO1 (5.0 ng) (n = 24). |
|
In vivo bromodeoxyuridine labeling of 1- and 3-day postfertilization rtn4 morphant embryos. (A) Overview of the bromodeoxyuridine (BrdU) pulse chase experiment. To maximize the labeling of cells entering the S-phase, a 1-hour BrdU pulse was applied at 1 dpf. Half of the embryos were fixed immediately after the pulse (B), (D), (F), (J), (K) and (L), and the other half were fixed 2 days later (C), (E), (G), (M), (N) and (O). Confocal maximum projections of midbrain and hindbrain sections showed a considerable amount of BrdU-labeled cells at 1 dpf (green) (B), (D), (F), (J), (K) and (L). Nuclei were counterstained with 42,6-diamidino-2-phenylindole (DAPI) (red). At 1 dpf, the presumptive tectum of rtn4a morphants (D), especially rtn4b morphants (F), is reduced in size relative to control embryos (B). (C), (E), (G), (M), (N) and (O) BrdU retention in the tectum and hindbrain at 3 dpf. In addition to the reduced tectum in 1-dpf rtn4a and rtn4b morphants, cells in the 3-dpf rtn4b morphants show strong BrdU signaling 2 days after the BrdU chase (G) and (O). Only weak and diffused BrdU signaling was detected in rtn4a (E) and (N) and control (C) and (M) groups. (H) and (P) Quantification of total cells (red) and proliferating cells (green) in the tectum and hindbrain at 1 dpf in rtn4a and rtn4b morphants and the control group. (I) Quantification of total cells in the tectum at 3 dpf (red). (Q) Quantification of total (red) vs. BrdU-positive cells (yellow) in analyzed areas of the hindbrain at 3 dpf in rtn4a and rtn4b morphants and the control group.*Melanocytes. Control (1 dpf; n = 8), rtn4a (n = 11), rtn4b (n = 12), control 2 dpf (n = 6), rtn4a (n = 6) and rtn4b (n = 14). Scale bar = 50 μm. |