- Title
-
Dre-miR-2188 Targets Nrp2a and Mediates Proper Intersegmental Vessel Development in Zebrafish Embryos
- Authors
- Soares, A.R., Reverendo, M., Pereira, P.M., Nivelles, O., Pendeville, H., Bezerra, A.R., Moura, G.R., Struman, I., and Santos, M.A.
- Source
- Full text @ PLoS One
|
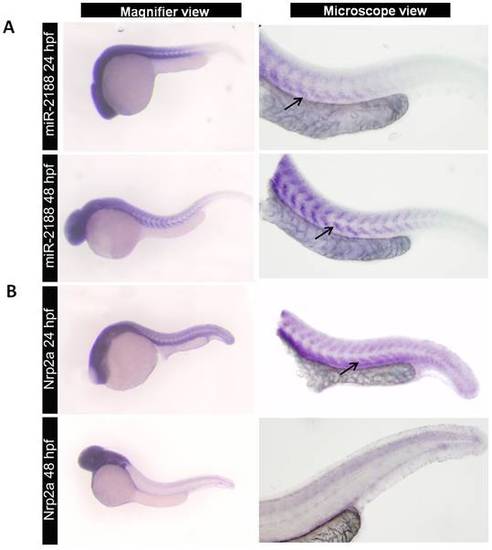
Expression of miR-2188 and Nrp2a in zebrafish embryos. A) ISH of miR-2188 at 24 hpf showed low expression on the trunck and tail, however it increased at 48 hpf between the somite boundaries. Arrows indicate hybridization signal. B) Nrp2a ISH at 24 and 48 pf. At 24hpf Nrp2a was predominantly expressed in the head and somitic regions, while at 48 hpf its expression was restricted to the brain and romboencephalon. There was an indirect correlation between miR-2188 and Nrp2a expression. Increasing miR-2188 levels through development were accompanied by decreasing Nrp2a expression between the somite boundaries. All embryos are in lateral view, anterior to left. Microscopic pictures were taken with 6,3x magnification. |
|
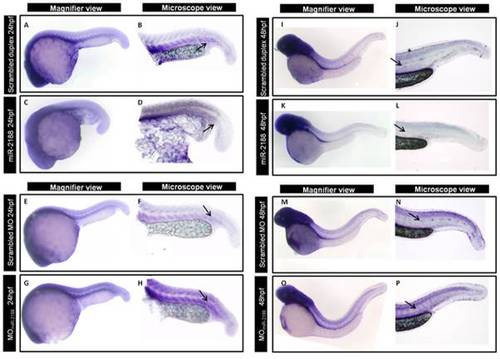
ISH of Nrp2a after miR-2188 deregulation. A) After injection of miR-2188 duplex, Nrp2a detection decreased between the somite boundaries at 24 hpf. On the other hand, in miR-2188 morphants, Nrp2a detection was more prounounced throughout the trunk and tail region, between the somite boundaries, when compared with the control. B) At 48 hpf and after miR-2188 overexpression, Nrp2a was not detected in the blood vessels or in the PCV when compared with the control embryos. On the other hand, miR-2188 morphants express Nrp2a in the trunck and tail, especially between the somite boundaries, which is not detected in 48 hpf control embryos. All embryos are in lateral view, anterior to left. Arrows indicate hybridization signal. Microscopic pictures were taken with a 6,3x magnification. |
|
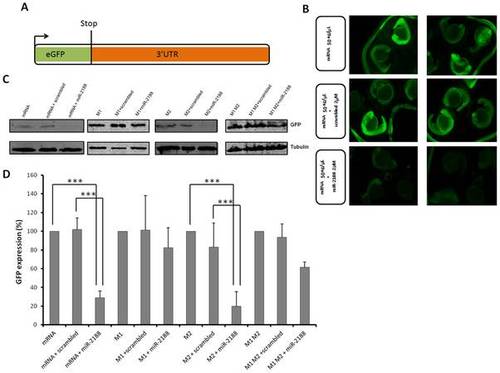
GFP sensor assay. A) The GFP gene was fused with the 3′UTR of Nrp2a that contained the putative binding sites of miR-2188. This construct was transcribed in vitro into capped mRNA prior to injection. B) Single cell embryos were injected with the GFP reporter (50 ng/μL) in the presence or absence of miR-2188 and scrambled duplexes. Fluorescence levels were observed at 24 hpf and representative embryos of each condition were photographed. Decreased fluorescence was observed in miR-2188 duplex injected embryos. C) Embryo lysates were prepared from 24 hpf embryos injected with the wild type GFP reporter or the mutated reporters in the presence or absence of miR-2188 and scrambled duplexes. Protein levels were determined by western blot analysis and β-tubulin was used as an internal control. D) Quantification of the reporter proteins (%) in the conditions tested using 3 biological replicates. Asterisks indicate conditions where GFP expression was significantly down regulated by miR-2188 relative to control conditions. miR-2188 injected embryos showed a statistically significant down regulation of GFP levels, indicating that Nrp2a 3′UTR is a true miR-2188 target. Co-injection of the M1 reporter with the miR-2188 duplex produced normal GFP levels, indicating that the BS-1 mutation abolished miR-2188 binding. On the other hand, a significant decrease in GFP expression was observed after co-injection of the M2 reporter with miR-2188, relative to the controls, indicating that BS-2 was not critical for miR-2188 binding. Data are mean +/stdev, p<0,005 (t test, unpaired), n>3. All lanes were normalized to the β-tubulin signal. |
|
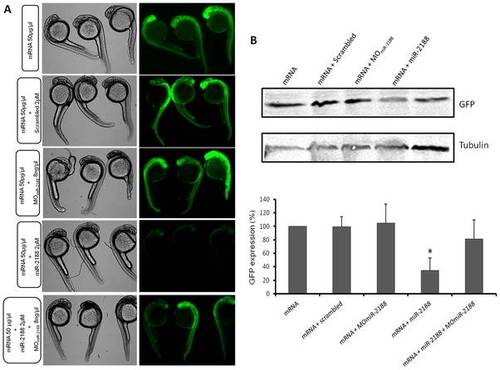
GFP rescue sensor assay. A) Single cell embryos were injected with the GFP reporter (50 ng/μL) in the presence or absence of MOmiR-2188, miR-2188 and scrambled duplexes or a mixture of both MOmiR-2188 and miR-2188 duplex. Fluorescence levels were observed at 24 hpf and representative embryos of each condition were photographed. Fluorescence was recovered in MOmiR-2188+ miR-2188 injected embryos, when compared to miR-2188 duplex injected embryos. B) Embryo lysates were prepared from 24 hpf embryos injected with the GFP reporter in the presence or absence of MOmiR-2188, miR-2188 and scrambled duplexes or a mixture of MOmiR-2188 and miR-2188. Protein levels were determined by western blot analysis and β-tubulin was used as an internal control. C) Quantification of the reporter proteins (%). Asterisks indicate conditions where GFP expression was significantly down regulated by miR-2188 relative to control conditions. miR-2188 injected embryos showed a statistically significant down regulation of GFP levels, indicating that Nrp2a 3′UTR is a true miR-2188 target. Co-injection of the MOmiR-2188 with the miR-2188 duplex resulted in the rescue of GFP fluorescence, further confirming that Nrp2a is a bona fide target of miR-2188. Data are mean +/stdev, p<0,005 (t test, unpaired), n>3. All lanes were normalized to the β-tubulin signal. |
|
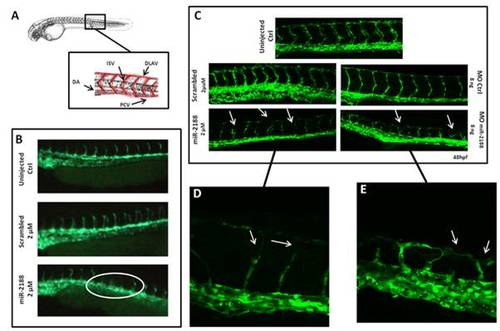
Analysis of blood vessel formation in Tg(flk1-GFP)s843 embryos. A) Representation of the zebrafish circulatory system showing the major structures (DLAV - Dorsal Longitudinal Anastomotic Vessel; ISV – Intersegmental Vessel; DA – Dorsal Aorta; PCV – Posterior Cardinal Vein). B) Visualization of 24 hpf embryo blood vessels using fluorescence microscopy. After miR-2188 duplex microinjection under developed and absent ISVs were observed, non injected and scrambled duplex injected embryos did not show such defects. C) Visualization of 48 hpf embryo blood vessels using confocal microscopy (20x). ISVs of miR-2188 injected embryos were thinner than those of non injected and scrambled duplex injected embryos and displayed ISV’s patterning defects (arrows). MOmiR-2188 injected embryos revealed DLAV defects and branching of ISVs (arrows). Images shown in D) and E) are 60x amplification images of miR-2188 duplex and miR-2188-MO injected embryos, respectively. PHENOTYPE:
|
|
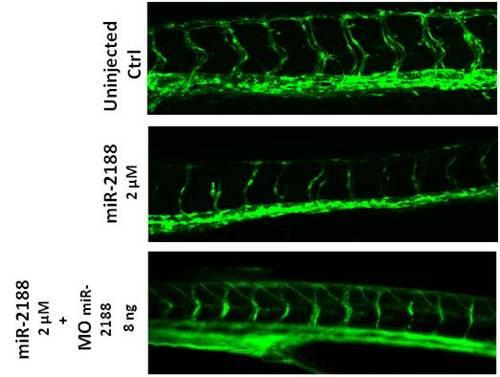
ISV patterning is rescued after injection of MomiR-2188 in embryos overexpressing miR-2188. Visualization of 48 hpf embryo blood vessels using confocal microscopy (20x). ISVs of miR-2188 injected embryos were thinner and displayed ISV patterning defects, when compared to non-injected embryos. Injection of MO miR-2188 in embryos over expression miR-2188 rescued the ISV patterning. |