- Title
-
Molecular cloning and embryonic expression of zebrafish PCSK5 co-orthologues: Functional assessment during lateral line development
- Authors
- Chitramuthu, B.P., Baranowski, D.C., Cadieux, B., Rousselet, E., Seidah, N.G., and Bennett, H.P.
- Source
- Full text @ Dev. Dyn.
|
Developmental expression of zebrafish PC5.1 and PC5.2. A: An ontogeny of expression for PC5.1 by whole mount in situ hybridization. Lateral (a) and dorsal (b) view of 18-hr post-fertilization (hpf) embryos revealed very discrete expression within the anterior and posterior part of the otic vesicle. At 24hpf (c), in addition to the anterior and posterior part of the otic vesicle, expression is observed within the anterior and posterior lateral line primordia. Lateral view of 36hpf (d) detected additional expression within the posterior lateral line neuromast L1 and L2. At 48hpf (e), 72hpf (f), and 96 hpf (g), PC5.1 is strongly localized in the otic vesicle and within the anterior and posterior lateral line neuromasts. The asterisks (A: e, f) represent neuromasts on the opposite side of the embryo. B: An ontogeny of expression for PC5.2 by whole mount in situ hybridization. At 18hpf (a, lateral; b, dorsal), ubiquitous expression with distinct regionalization within the somites, Kupffer′s vesicle, and in the tail bud is observed. Lateral view of 24hpf (c) shows continued expression within the somites and CNS with additional expression within the otic vesicle and pronephric duct. At 36hpf (d) and 48hpf (e, lateral view; f, dorsal view), PC5.2 expression is found within the pharyngeal region, fin bud, CNS, otic vesicle, and pronephric duct. Lateral (g, i) and dorsal (h, j) views of 72hpf and 96hpf, respectively, show a higher level of expression within the liver and gut. C: Localization of immunoreactive PC5.1 within the hair cells and supporting cells of the neuromast. Zebrafish embryos at 72hpf were stained with anti-PC5.1 antibody. Bright field image of neuromast (a), localization of PC5.1 within the hair cells (b), and localization of PC5.1 within the supporting cells (c) were visualized using confocal microscopy. A: Aov, anterior part of the otic vesicle; pov, posterior part of the otic vesicle; allp, anterior lateral line primordium; pllp, posterior lateral line primordium; alln, anterior lateral line neuromast; D1 and D2, dorsal lateral line neuromasts; L1-L8, posterior lateral line neuromasts. B: som, somites; Kv, kupffer′s vesicle; tb, tail bud; ov, otic vesicle; pd, pronephric duct; ba, branchial arches; fb, fin bud; c, cerebellum; t, tectum; hb, hindbrain. EXPRESSION / LABELING:
|
|
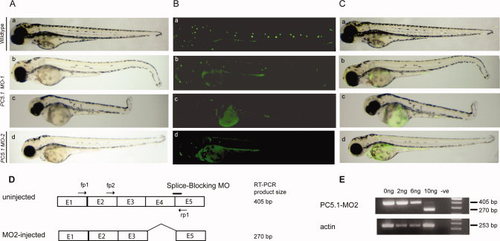
Morpholino-based translation inhibition disrupts formation of the lateral line. A: Phenotypic consequences of PC5.1 knockdown. MO-1 injected embryos (b, c) and MO-2 injected embryos (d) at 72 hpf displayed curved posterior body plan compared to wild type (a). B, C: Loss of functional neuromasts in PC5.1 Morphant detected by 4-Di-2-Asp staining. Comparison of WT (B, C: a) MO-1-injected embryos at 72hpf indicated either reduced (B, C: b) or complete absence (B,C: c) of posterior lateral line neuromasts. MO-2-injected embryos at 72 hpf showed either reduced (B,C: d) posterior lateral line neuromast or severe (data not shown) phenotypes. Knockdown of PC5.1 also resulted in circular swimming pattern and lack of a normal touch response. D: Altered splicing of zebrafish PC5.1 resulting from the use of morpholino, MO-2, to target a splice site boundary. The splice-blocking morpholino MO-2 overlaps the exon-intron boundary at the 32 splice junction of exon 4. The position of the forward and reverse primers used for diagnostic RT-PCR is indicated. The expected sizes of the RT-PCR products are indicated. E1 to E5 represents exon 1 to exon5 of PC5.1. E: RT-PCR of two 24hpf embryos either uninjected or injected with 2, 6, or 10 ng of MO-2. The 405-bp product of the normal-spliced PC5.1 fragment was detected in the uninjected embryos and in the 2- and 6-ng-injected embryos and to a much lesser extent in the 10-ng-injected embryos. The strong 270-bp product representing mRNA PC5.1 deleted from exon 4 was observed following RT-PCR of the embryos injected with 10 ng of MO-2. No template (-ve) and actin were used as negative and positive controls, respectively. PHENOTYPE:
|
|
Role of PC5.1 in lateral line neuromasts development as revealed by alkaline phosphatase staining of larvae injected with either PC5.1 MO-1 or with PC5.1 and p53 morpholino co-injection. The injected larvae at 72–96 hpf were labeled with Di-Asp. Then the larvae were fixed and stained for alkaline phosphatase activity. Both the larvae injected with PC5.1 MO-1 (B) and co-injected with PC5.1 and p53 morpholinos (C, D) show a marked decrease in the number of labeled neuromasts when compared to wild-type larvae (A). Individual neuromasts of the posterior lateral line are indicated by arrows. PHENOTYPE:
|
|
In situ hybridization of CB701, a primordium marker, confirms that the primordium of the PC5.1-deficient larvae fails to deposit lateral line neuromasts. Expression of CB701 mRNA using an anti-sense RNA probe shows localisation within the lateral line neuromasts of wild-type larvae (A). In the larvae subjected to PC5.1 knockdown, only a few neuromasts were labeled for CB701 (B). |
|
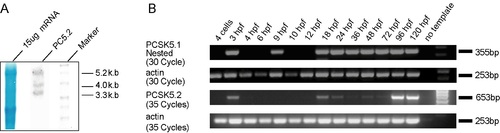
Northern blot, RT-PCR, and Western blot analyses of zebrafish PCSK5 genes. A: Northern blot analysis using strand-specific cRNA probes was performed to confirm the size of the cloned cDNA sequences of PC5.1 and PC5.2. Fifteen micrograms of mRNA from whole adult zebrafish was separated by electrophoresis on 1% agarose/formaldehyde gels and transferred to nitrocellulose membranes. The membranes were hybridized with non-isotopically labeledPC5.1 and PC5.2 cRNA probes and exposed to X-ray film. Methylene Blue staining of 28S and 18S rRNA (lane 1) served as loading controls. PC5.2 detected three transcripts of approximately 3.3, 4.0, and 5.2 kb in size, respectively. Note that the 3.3-kb band is in agreement with cloned sequences for this mRNA (3,319 bp). B: PC5.1 transcript is detected as early as 3 hr post-fertilization (hpf) and throughout all stages of development that follow 18hpf. The PC5.1-specific RT-PCR product was run on an agarose gel and transferred to nitrocellulose membrane and developed using a gene-specific RNA probe. This result demonstrates that PC5.1 transcription occurs, weakly, at 4-cell stage and indicates its maternal expression (data not shown) and is first detected by RT-PCR at 3hpf, 18hpf, and all the stages examined. In contrast to PC5.1, PC5.2 is expressed at varying levels. Numbers of cycles used for the PCR are indicated. No template (-) and actin were used as negative and positive controls, respectively. Gene-specific primers and amplicon sizes are listed in Supp. Figure S6. C: Western blot analysis using protein extracts from sets of 10 embryos each at 24hpf employing 10% SDS-PAGE. A 75-kDa protein product was detected for PC5.1 and 150- and 25-kDa species for PC5.2. Faint signals are also detected at about 50 kDa for both PC5.1 and PC5.2, suggesting the presence of splice variants. |