- Title
-
Foxd3 is an Essential Nodal-Dependent Regulator of Zebrafish Dorsal Mesoderm Development
- Authors
- Chang, L.L., and Kessler, D.S.
- Source
- Full text @ Dev. Biol.
|
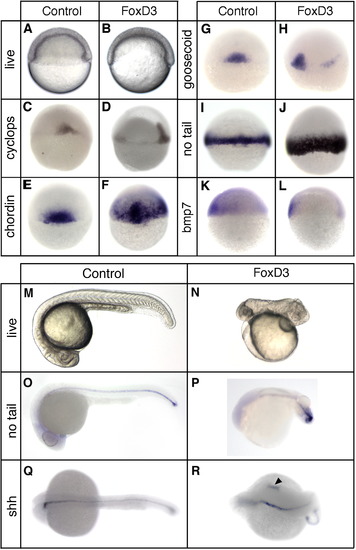
Foxd3 induction of dorsal mesoderm and axial dorsalization. At the one-cell stage embryos were injected with 25 pg of foxd3 mRNA and analyzed at the shield stage (6 hpf) (A–L) or at 24 hpf (M–R). (A, B) Live embryos (lateral views, dorsal right) showing the shield and blastoderm structure in uninjected (A) and foxd3-injected (B) embryos. In situ hybridization of uninjected (C, E, G, I, K) and foxd3-injected (D, F, H, J, K) embryos showing expression of cyclops (C, D), chordin (E, F), goosecoid (G, H), no tail (I, J), and bmp7 (K, L). Views shown are dorsal (C, E, F, G, I, J), dorsal lateral (D, H), or lateral with dorsal right (K, L). Uninjected (M, O, Q) and foxd3-injected (N, P, R) embryos at 24 hpf. Shown are live embryos (M, N), and embryos analyzed by in situ hybridization for no tail (O, P) or sonic hedgehog (shh) (Q, R) expression. Views are lateral with anterior left (M–P) or dorsal with anterior left (Q, R). Ectopic expression of sonic hedgehog was observed in foxd3-expressing embryos (R, arrowhead). |
|
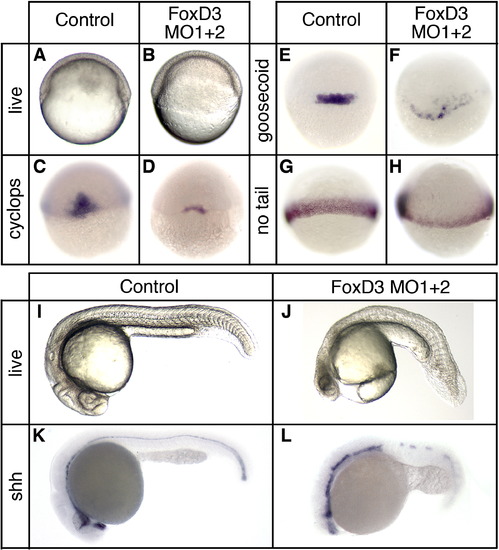
Foxd3 is essential for mesodermal development and axis formation. At the one-cell stage embryos were injected with a mixture of two foxd3-specific antisense morpholino oligonucleotides (MO1 + 2, total dosage 20 ng) and analyzed at the shield stage (6 hpf) (A–H) or at 24 hpf (I–L). (A, B) Live embryos (lateral views, dorsal right) showing the shield and blastoderm structure in uninjected (A) and Foxd3 knockdown (B) embryos. In situ hybridization of uninjected (C, E, G) and FoxD3 knockdown (D, F, H) embryos showing expression of cyclops (C, D), goosecoid (E, F), and no tail (G, H) (dorsal views). Uninjected (I, K) and Foxd3 knockdown (J, L) embryos at 24 hpf. Shown are live embryos (I, J), and embryos analyzed by in situ hybridization for sonic hedgehog (shh) (K, L) expression (lateral views with anterior left). A foxd3 oligonucleotide with multiple mismatches did not produce mesodermal or axial phenotypes at a dosage (20–40 ng) equal to or greater than the perfect match oligonucleotides (data not shown). See Supplementary materials for rescue experiments (Fig. S2). |
|
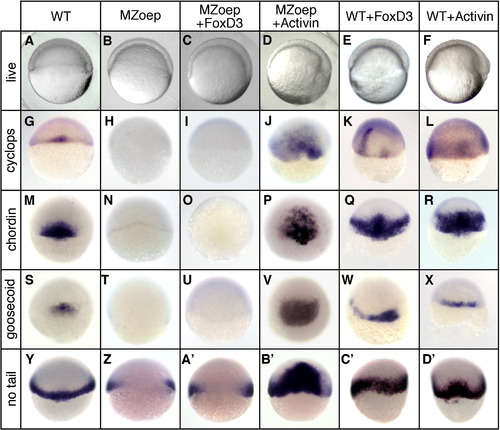
Foxd3 induction of mesoderm is dependent on an active Nodal signaling pathway. Wild-type (WT) or MZoep mutant embryos were injected at the one-cell stage with zebrafish foxd3 (25 pg) or Xenopus ActivinΒB (25 pg) mRNA and analyzed at the shield stage (6 hpf). (A–F) Live embryos (lateral views, dorsal right) showing the shield and blastoderm structure in uninjected (A, B), foxd3-injected (C, E), and Activin-injected (D, F) embryos. In situ hybridization showing expression of cyclops (G–L), chordin (M–R), goosecoid (S–X), and no tail (Y–D′) (dorsal views). |
|
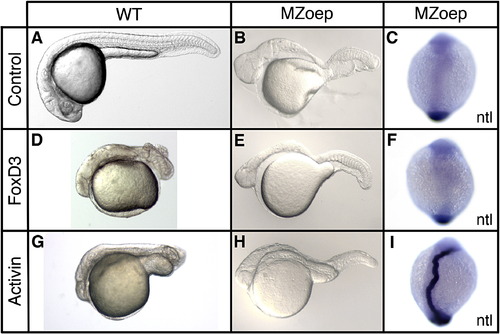
Foxd3 regulation of axis formation is dependent on the Nodal signaling pathway. Wild-type (WT) or MZoep mutant embryos were injected at the one-cell stage with zebrafish foxd3 (25 pg) or Xenopus ActivinΒB (25 pg) mRNA and analyzed at the 24 hpf stage (A, B, D, E, G, H) or early somitogenesis stage (11 hpf) (C, F, I). Uninjected (A, B), foxd3-injected (D, E), and Activin-injected (G, H) wild-type (A, D, G) and MZoep (B, E, H) embryos at 24 hpf are shown (lateral views of live embryos with anterior left). In situ hybridization showing notochord expression of no tail (ntl) (C, F, I) in uninjected (C), foxd3-injected (F), and Activin-injected (I) MZoep embryos at early somitogenesis stage (11 hpf) (dorsal views with anterior up). |
|
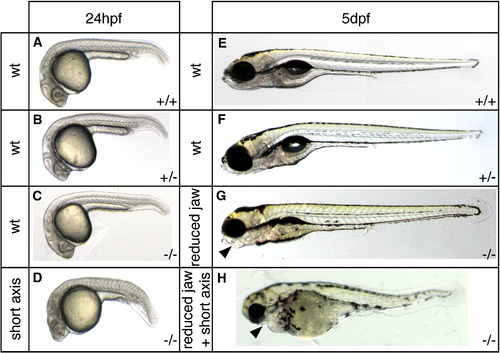
Axis formation defects in sym1 embryos. Heterozygous sym1 adults were crossed and axial development of progeny was analyzed at ∼ 24 hpf and ∼ 5 dpf. Representative samples of the two phenotypic classes observed at 24 hpf (n = 119): wild-type (90%) (A, B, C), and axial defects (10%) (D), and representative samples of the three phenotypic classes observed at 5 dpf (n = 494): wild-type (77%) (E,F), craniofacial defects (14%) (G), and craniofacial defects together with axial defects (9%) (H). Genotyping analysis indicated that at 24 hpf (A–D) the wild-type phenotypic class consisted of genotypically wild-type (+/+), sym1 heterozygotes (+/-), and sym1 homozygotes (-/-), while axial defect class consisted only of sym1 homozygotes (-/-) . At 5 dpf (E–H) the wild-type phenotypic class consisted of both genotypically wild-type (+/+) and sym1 heterozygotes (+/-), while the craniofacial defect and the craniofacial with axial defect classes consisted only of sym1 homozygotes (-/-). Arrowhead indicates reduced jaw structures in the craniofacial defect class (G) and in the craniofacial defects together with axial defect class (H). Phenotypic class is indicated to the left of each panel and genotype is shown on the bottom right of each panel. |
|
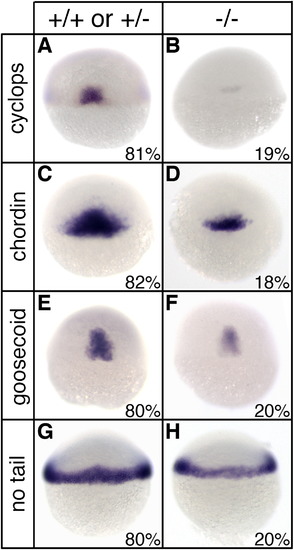
Reduced mesodermal gene expression in sym1 mutants. Heterozygous sym1 adults were crossed and progeny were examined for mesodermal gene expression by in situ hybridization at shield stage (6 hpf). For cyclops (A, B), chordin (C, D), goosecoid (E, F), and no tail (G, H), two phenotypic classes were observed: wild-type (A, C, E, G) and reduced gene expression (B, D, F, H). For each mesodermal gene analyzed, distribution of progeny into the two classes was ∼ 80% for the wild-type class and ∼ 20% for the reduced class (exact distribution for each gene is indicated in lower right of each panel). Representative embryos were selected for genotyping analysis following in situ hybridization and this indicated that the wild-type phenotypic class consisted of both genotypically wild-type (+/+) and sym1 heterozygotes (+/-), while the reduced gene expression class consisted only of sym1 homozygotes (-/-). Dorsal views are shown. |
|
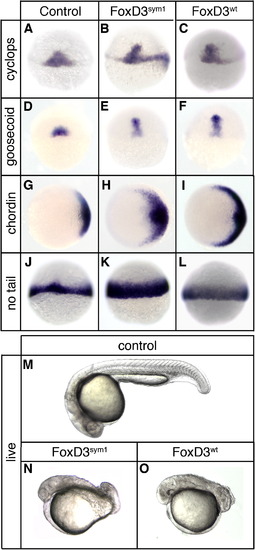
sym1 retains mesoderm induction and axis dorsalizing activity. At the one-cell stage embryos were injected with 150 pg of sym1 mRNA (Foxd3sym1) (B, E, H, K, N) or 25 pg of wild-type foxd3mRNA (Foxd3wt) (C, F, I, L, O) and analyzed at the shield stage (6 hpf) (A–L) or at 24 hpf (M–O). In situ hybridization at shield stage of uninjected (A, D, G, J), sym1-injected (B, E, H, K), and foxd3-injected (C, F, I, L) embryos showing expression of cyclops (A–C), goosecoid (D–F), chordin (G–I), and no tail (J–L). Views shown are dorsal (A–F, J–L) or animal with dorsal right (G–I). Uninjected (M), sym1-injected (N), and foxd3-injected (O) embryos at 24 hpf. Shown are live embryos (lateral views with anterior left). |
|
Zebrafish Foxd3 induces mesoderm in Xenopus animal explants. At the one-cell stage, Xenopus embryos were injected in the animal pole with 100 pg of Xenopus foxd3 (xFoxD3, lane 2) or zebrafish foxd3 (zFoxD3, lane 3), explants were prepared at the late blastula stage (stage 9) and analyzed by RT-PCR at the tailbud stage (stage 25) for the expression of Muscle Actin (somitic muscle) and Collagen II (notochord). EF1α is a control for RNA recovery and loading, intact embryos (Embryo, lane 4) served as a positive control and an identical reaction without reverse transcriptase controlled for PCR contamination (Embryo-RT, lane 5). |
|
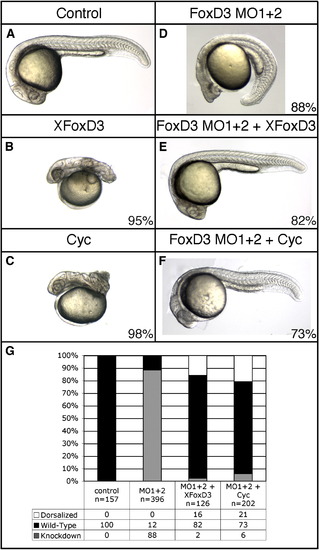
Rescue of Foxd3 knockdown by Xenopus foxd3 or cyclops. At the one-cell stage, wild-type embryos were injected with a mixture of foxd3MO1 and foxd3MO2 (MO1 + 2, total dosage 20 ng) alone (D), or in combination with 30 pg of Xenopus foxd3 (XFoxD3) (E) or 20 pg of cyclops (Cyc) (F) mRNA, and axial development was assessed at 24 hpf (A–F). Embryos were phenotypically classified as knockdown (D), wild-type (E, F) or dorsalized (not shown), and these classes are quantified in (G). Knockdown embryos have reduced head structures, reduction of trunk mesoderm, and expanded tail somites (D), while rescued embryos are normal (E) or near normal (F) in all aspects of axis formation. Injection of MO1 + 2 alone resulted in 88% (n = 396) of embryos with the knockdown phenotype, while 82% (n = 126) of Xfoxd3-injected and 73% (n = 202) of cyclops-injected embryos were phenotypically wild-type. A minority of knockdown embryos rescued by injection of Xfoxd3 (16%) or cyclops (21%) mRNA displayed a dorsalized body axis. Injection of Xfoxd3 or cyclops mRNA alone strongly dorsalized control embryos (B, C). Shown are lateral views, anterior left, of live embryos. |
|
Antivin blocks the mesodermal activity of Foxd3. At the one-cell stage, wild-type embryos were injected with Antivin (10 pg) (B, F, J, N), foxd3 (25 pg) (D, H, L, P), or a mixture of both mRNAs (C, G, K, O). At the shield stage (6 hpf) live embryos were examined for shield and blastoderm structure (A–D), and fixed embryos were analyzed by in situ hybridization for cyclops (E–F), goosecoid (I–L), and no tail (M–P) expression. Shown are lateral views with dorsal right (A–D), dorsal views (E–L), and animal views with dorsal right (M–P). |
Reprinted from Developmental Biology, 342(1), Chang, L.L., and Kessler, D.S., Foxd3 is an Essential Nodal-Dependent Regulator of Zebrafish Dorsal Mesoderm Development, 39-50, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.